Abstract
Residents of Chikusei City, aged 40–74 years, underwent systemic and ophthalmological screening, and participants with diabetes were included in this analysis. Dietary intake was assessed using a food frequency questionnaire and calculated as a percentage of the total energy. The presence of diabetic retinopathy (DR) was defined as Early Treatment Diabetic Retinopathy Study levels ≥ 20 in either eye. The association between dietary fatty acid intake and DR has been examined in a cross-sectional study. Among the 647 diabetic participants, 100 had DR. The mean total fat and saturated fatty acid (SFA) intakes were 22.0% and 7.3% of the total energy intake, respectively. After adjusting for potential confounders, the highest quartiles of total fat and SFA intake were positively associated with the presence of DR compared with the lowest quartiles (odds ratios (95% confidence intervals), 2.61 (1.07–6.39), p for trend = 0.025, and 2.40 (1.12–5.17), p for trend = 0.013, respectively). No significant associations were found between DR prevalence and monounsaturated or unsaturated fatty acid intake. These results suggest that a high intake of fat and SFA may affect the development of DR, even in individuals whose total fat intake is generally much lower than that of Westerners.
Similar content being viewed by others
Introduction
Diabetic retinopathy (DR) is the leading cause of visual impairment in working adults1, and is also the most common microvascular complication of diabetes. According to a meta-analysis of 59 population-based studies, the global prevalences of DR and vision-threatening DR were 22.27% and 6.17%, respectively, with an estimated 103.12 million individuals living with DR and 28.54 million living with vision-threatening DR in 20202. The major risk factors for DR include prolonged duration of diabetes and poor control of glycemia, blood pressure, and lipids1,3.
Nutrition plays an important role in the pathogenesis and prevention of ocular diseases. Dietary intake of n-3 polyunsaturated fatty acids (PUFA) by fish is associated with a lower risk of age-related macular degeneration (AMD)4,5,6,7. A meta-analysis suggested that n-3 PUFA intake reduces the risk of late AMD, whereas fish consumption reduces the risk of both late and early AMD8. Meanwhile, we recently reported that a higher intake of saturated fatty acid (SFA) in a population with low mean SFA intake was associated with a lower risk of early AMD9, suggesting that the association between fatty acid intake and AMD could differ among populations with different genetic backgrounds or dietary patterns.
Although the association between fatty acid intake and the risk of developing diabetes has not been fully elucidated, several studies have reported that SFA intake is a risk factor for developing diabetes, whereas PUFA intake may reduce this risk10,11,12,13. In addition, growing evidence suggests that PUFA intake has a beneficial effect on complications associated with diabetes. Omega-3 PUFA supplementation favorably modified cardiometabolic biomarkers, lipids, glycemia, and pro-inflammatory cytokines in type 2 diabetes14. A randomized controlled trial showed that the administration of n-3 PUFA supplements attenuated the progression of albuminuria in individuals with type 2 diabetes mellitus and a history of coronary artery disease15. The National Health and Nutrition Examination Survey in the United States reported that dietary intake of n-6 PUFA and linolenic acid is associated with a lower risk of peripheral neuropathy16. Asian populations consume smaller amounts of SFA-containing foods than Western populations17,18, and a meta-analysis reported ethnic differences in the relationship between insulin sensitivity and response19. However, studies examining the association between fatty acid intake and DR have shown inconsistent results20,21,22,23,24, and such studies have not been conducted among Asians.
Since dyslipidemia is a potential risk factor for DR25,26,27, dietary intake of fatty acids may affect lipid metabolism and the pathogenesis of DR. Therefore, we aimed to examine the cross-sectional association between dietary fatty acid intake and the prevalence of DR in participants with diabetes in a Japanese population-based cohort, the Japan Public Health Center-based Prospective Study for the Next Generation (JPHC-NEXT) Eye Study.
Results
In total, 647 and 100 participants were identified as having diabetes and DR, respectively. The characteristics of the 647 patients with DM are presented in Table 1. The mean total energy intake was 2292.5 kilocalories (kcal), and the total fat and SFA intakes were 22.0% and 7.3% of the total energy intake (% energy), respectively. There was no significant difference in total energy intake between patients with and without DR. The intakes of total fat, SFA, PUFA, and n6-PUFA were significantly higher in patients with DR than in those without DR. Conversely, there was no significant difference in the intake of MUFA and n3-PUFA. The patients with and without DR were similar in terms of age, sex, body mass index (BMI), smoking status, hypertension, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and intake of α-tocopherol, β-carotene, vitamin C and vitamin D. Patients with DR had higher glycated hemoglobin (HbA1c) levels and protein intake, lower serum triglyceride (TG) levels, less dyslipidemia, and were less likely to be current drinkers.
Associations between fatty acid intake and the prevalence of DR
The associations between specific types of dietary fatty acid intake and DR are shown in Table 2. After adjusting for age, sex, total energy intake, smoking status, alcohol intake, HbA1c, systolic blood pressure (SBP), dyslipidemia, BMI, creatinine, and vitamin intakes (α-tocopherol, β-carotene, vitamin C and vitamin D), the highest quartiles of the total fat and SFA intake were positively associated with the presence of DR compared with the lowest quartiles (odds ratio [OR], 2.61; 95% confidence interval [CI], 1.07–6.39; P for trend = 0.025; and OR, 2.40; 95% CI 1.12–5.17; P for trend = 0.013, respectively) (Table 2, Model 3) (Fig. 1). No significant associations were found between the prevalence of DR and the intakes of MUFA, PUFA, n3-PUFA, or n6-PUFA.
Estimated probabilities of presenting with diabetic retinopathy. Estimated probabilities of presenting with diabetic retinopathy by total fat intake (a) and saturated fatty acid intake (b). Logistic regression models were adjusted for age, sex, total energy, smoking status, alcohol intake, HbA1c, SBP, dyslipidemia, BMI, creatinine and intakes of α-tocopherol, β-carotene, vitamins C and D. DR, diabetic retinopathy.
Further subgroup analyses of the association between SFA intake and DR were performed and stratified according to well-controlled (HbA1C < 7.0%) and poorly controlled (HbA1C ≥ 7.0%) diabetes. In patients with well controlled diabetes, an increased SFA intake tended to be associated with an increased odds of developing DR (model 3: p for trend = 0.095). Among the poorly controlled patients, the association was weaker, but a similar tendency was observed.
Discussion
We analyzed a Japanese cohort of participants with diabetes and found that the intakes of total fat and SFA were positively associated with the presence of DR. Previous studies examining the association between SFA intake and DR have been limited, with inconsistent results20,22. A case–control study of 294 patients with type 2 Diabetes in Spain reported that the intake of trans fats and SFA was not associated with the prevalence of DR22. Meanwhile, a cross-sectional study of 379 patients with diabetes in Australia showed that SFA intake was associated with the prevalence of DR when glycemic control was good (HbA1C < 7.0%), whereas no association was evident when glycemic control was poor (HbA1C > 7.0%)20. The authors speculated that the harmful effects of hyperglycemia may counteract the influence of fatty acid intake in patients with poorly controlled diabetes. In the current study, patients with diabetes had relatively good glycemic control, with a mean HbA1c of 7.0%, which may partly explain the association between SFA intake and DR.
The role of SFA in health has been discussed extensively. The World Health Organization28 advocates that total fat should not exceed 30% of the total energy intake29,30,31, and that SFA intake should be less than 10%, with a shift in fat consumption away from SFA to unsaturated FA31 for the general population. Asians consume smaller amounts of SFA-containing foods than Western populations17,18.According to the US National Health and Nutrition Examination Survey, the mean intakes of total fat and SFA were 36.2% and 11.7% of the total energy intake, respectively, in American individuals aged 20 years or older32. Meanwhile, according to the National Health and Nutrition Survey in Japan, the mean intakes of total fat and SFA are 28.7% and 8.4% of the total energy intake in Japanese individuals aged 20 years or older33, respectively. In the present study, the mean intakes of total fat and SFA were 22.0% and 7.3% of the total energy intake, respectively, in patients with diabetes aged 40 years or older. Our results suggest that high fat intake may affect the prevalence of DR, even in patients with diabetes mellitus whose total fat intake is below the recommended levels.
No significant association was observed between PUFA intake and DR prevalence in the present study. Previous studies examining the association between the prevalence of DR and intake of PUFA and n-3 PUFA have shown inconsistent results20,21,22,23,24. A cross-sectional study in Australia reported that PUFA intake was inversely associated with the prevalence of DR when individuals with diabetes had good glycemic control20. The Prevención con Dieta Mediterránea (PREDIMED) trial, a randomized controlled study examining the effect of the Mediterranean diet on 3482 patients with type 2 diabetes, found that patients who consumed n-3 PUFA at or above the recommended dose for the prevention of cardiovascular disease (500 mg/day) had an approximately 50% lower risk of developing DR with visual impairment21. In the present study, the mean energy-adjusted n-3 PUFA intake was 2.3 g/day. We speculate that the lack of association between n-3 PUFA intake and the prevalence of DR in the present study may be partly due to the high consumption of n-3 PUFA over a potential threshold9,34. Further studies are required to clarify the association between PUFA intake and risk of DR.
Furthermore, in the current study, participants with DR exhibited lower serum triglyceride (TG) levels and a lower prevalence of dyslipidemia and were less likely to be current drinkers. There have been conflicting results regarding the association of serum TG levels and dyslipidemia with DR. Yao et al. reported that TG levels were inversely associated with DR, consistent with our study results35. However, a meta-analysis found no association of TG level or dyslipidemia with DR1. Several studies have reported a positive or negative association between alcohol intake and DR, but one meta-analysis found no such association36. Therefore, the results of this study do not necessarily contradict those of previous studies.
The strengths of this study include the use of standardized grading protocols to diagnose DR, as assessed by ophthalmologists, including retinal specialists, and the use of detailed questionnaires to collect lifestyle and medical history data. The validated the Food Frequency Questionnaire (FFQ) allowed the calculation of the intake of specific fatty acids. Our study has several limitations. First, the design of this study was cross-sectional, and we were unable to detect temporal information regarding associations. Second, we were unable to gather detailed information regarding diabetes status, including its duration, owing to the limitations of the data derived from a population-based cohort. Third, the correlation coefficients between fatty acid intake calculated from the FFQ and dietary records were lower in women than in men, which may have attenuated the association between fatty acid intake and DR in women. Fourth, in the current study, trans fatty acids and cellulose could not be analyzed due to a lack of data, and no studies to date have reported a certain relationship between trans fatty acid or cellulose intake and DR37,38. Finally, due to multicollinearity concerns, we did not include protein in the model, and no studies indicate a significant association between protein intake and DR after adjustment for confounders38. Nonetheless, it is crucial to recognize the potential confounding effect of nutrients. Therefore, future studies should investigate this possibility to gain further insights.
In summary, we found that total fat and SFA intakes were positively associated with the presence of DR. Our findings suggest that higher fat intake may affect the prevalence of DR, even in individuals whose total fat intake is generally much lower than that of Westerners and those who are more susceptible to developing diabetes. Although prospective longitudinal studies are needed to confirm this observation, these findings would enable us to understand the potential role of dietary fatty acid intake in the improved management of DR.
Population and methods
Study population
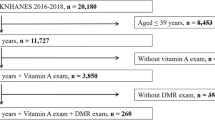
The JPHC-NEXT Eye Study is an ancillary study conducted as part of the JPHC-NEXT Study protocol39. Residents of Chikusei City, Japan, aged 40–74 years participated in this systemic and ophthalmological survey. The present study included 7090 individuals who participated in the survey between 2013 and 2015, of whom 5691 (80.3%) aged 40–74 years completed the FFQ. After excluding 14 participants because of missing or poor-quality fundus images, 647 participants with diabetes were included in the analysis.
This study was conducted in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects, Japan, and was approved by the Medical Ethics Committees of the School of Medicine, Keio University, Tokyo; the University of Tsukuba, Ibaraki; the University of Osaka, Osaka; and the National Cancer Center, Tokyo. Written informed consent was obtained from all the participants.
Data and sample collection
Non-mydriatic fundus photographs of both eyes were taken using a 45° non-mydriatic fundus camera (Canon CR-1, Canon Inc., Tokyo, Japan) as part of eye screening. The images were centered on the optic disc and macula.
Blood samples were collected to measure serum glucose (fasting or nonfasting), HbA1c (%), TC (mmol/L), HDL-C (mmol/L), LDL-C (mmol/L), and TG (mmol/L). The non-fasting state was defined as fasting for < 8 h after the last meal. Diabetes was defined as the use of antidiabetic medication, or a fasting serum glucose ≥ 7.0 mmol/L or non-fasting serum glucose ≥ 11.1 mmol/L, or HbA1c ≥ 6.5% (National Glycohemoglobin Standardization Program)40. Dyslipidemia was defined as the use of lipid-lowering medication, or LDL-C ≥ 3.6 mmol/L or HDL-C < 1.0 mmol/L, or TG ≥ 1.7 mmol/L41. Blood pressure (BP) was measured twice on the right upper arm while the participant was seated. Mean values were used for the analysis. Hypertension was defined as the use of blood pressure medication, or a systolic BP ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg42. BMI was calculated as weight (kg) divided by height squared (m2).
Grading of fundus photographs for DR
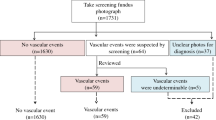
Any DR was defined as an Early Treatment Diabetic Retinopathy Study (ETDRS) levels ≥ 20 in either eye. The prevalence of DR was determined by two ophthalmologists who were blinded to the participants’ clinical data (T.K., H.T., E.Y., Y.K., K.M., or H.K.). In cases of disagreement, the diagnosis was made by a retinal specialist (Y.T. or N.O.).
Dietary assessment
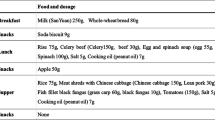
The dietary intake was evaluated using the long-form FFQ in the JPHC-NEXT study43. Briefly, the long-form FFQ comprises 172 food and beverage items and nine frequency categories, ranging from “almost never” to “seven or more times per day,” or to “10 or more glasses per day” for beverages. The questionnaire inquired about the usual consumption of the listed foods and beverages during the previous year. The nutrients, including fatty acids, in each food item were estimated using the fifth version of the Japan Food Table44. The nutrient intake was calculated by multiplying the frequency of consumption by the estimated intake for each food and summing across all items. Fatty acid intake was calculated as the percentage of total energy intake (nutrient density), and vitamin intakes were expressed as rates per 1000 kcal. They were divided into quantiles for further analysis. The validity of the FFQ for assessing fatty acid intake was confirmed using 12-day weighed food records (12d-WFR).43 The Spearman’s correlation coefficients for correlation between the energy-adjusted intake of fatty acids calculated from FFQ and dietary records ranged from 0.38 (for n-3 PUFA) to 0.55 (for MUFA) for men and from 0.21 (for MUFA) to 0.46 (for SFA) for women43, indicating moderate validity for fatty acids.
Statistical analysis
Baseline characteristics were calculated for the overall sample and subgroups stratified according to the presence of DR (Table 1). Differences in basic characteristics between patients with and without DR were tested using the Wilcoxon rank-sum test for continuous variables and the chi-square test for categorical variables. Associations between fatty acid intake and DR prevalence were examined using multivariable logistic regression models and expressed as odds ratios (ORs) with 95% confidence intervals (CIs). In the first model, we adjusted for age, sex, and total energy intake, in the second model further for smoking status (current or non-current smokers) and alcohol intake (current or non-current drinkers), HbA1c, SBP, dyslipidemia, BMI, and creatinine, and the third model further for intakes of α-tocopherol, β-carotene, vitamins C and D. Statistical significance was set at P < 0.05. significant. All statistical analyses were performed using SAS for Windows, version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Data availability
The data that support the findings of this study are available upon request from the corresponding author, MS, or KY. The data are not publicly available because they contain information that can compromise the privacy of the research participants.
References
Yau, J. W. et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35, 556–564. https://doi.org/10.2337/dc11-1909 (2012).
Teo, Z. L. et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 128, 1580–1591. https://doi.org/10.1016/j.ophtha.2021.04.027 (2021).
Lim, L. S. & Wong, T. Y. Lipids and diabetic retinopathy. Expert. Opin. Biol. Ther. 12, 93–105. https://doi.org/10.1517/14712598.2012.641531 (2012).
Seddon, J. M., George, S. & Rosner, B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: The US Twin Study of Age-Related Macular Degeneration. Arch. Ophthalmol. 124, 995–1001. https://doi.org/10.1001/archopht.124.7.995 (2006).
SanGiovanni, J. P. et al. The relationship of dietary lipid intake and age-related macular degeneration in a case-control study: AREDS Report No. 20. Arch. Ophthalmol. 125, 671–679. https://doi.org/10.1001/archopht.125.5.671 (2007).
Seddon, J. M., Cote, J. & Rosner, B. Progression of age-related macular degeneration: Association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch. Ophthalmol. 121, 1728–1737. https://doi.org/10.1001/archopht.121.12.1728 (2003).
Chiu, C. J., Milton, R. C., Klein, R., Gensler, G. & Taylor, A. Dietary compound score and risk of age-related macular degeneration in the age-related eye disease study. Ophthalmology 116, 939–946. https://doi.org/10.1016/j.ophtha.2008.12.025 (2009).
Chong, E. W., Kreis, A. J., Wong, T. Y., Simpson, J. A. & Guymer, R. H. Dietary omega-3 fatty acid and fish intake in the primary prevention of age-related macular degeneration: A systematic review and meta-analysis. Arch. Ophthalmol. 126, 826–833. https://doi.org/10.1001/archopht.126.6.826 (2008).
Sasaki, M. et al. dietary saturated fatty acid intake and early age-related macular degeneration in a Japanese population. Invest. Ophthalmol. Vis. Sci. 61, 23. https://doi.org/10.1167/iovs.61.3.23 (2020).
Guasch-Ferre, M. et al. Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the Prevencion con Dieta Mediterranea (PREDIMED) study. Am. J. Clin. Nutr. 105, 723–735. https://doi.org/10.3945/ajcn.116.142034 (2017).
Wang, L. et al. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 78, 91–98. https://doi.org/10.1093/ajcn/78.1.91 (2003).
Hodge, A. M. et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: Interpreting the role of linoleic acid. Am. J. Clin. Nutr. 86, 189–197. https://doi.org/10.1093/ajcn/86.1.189 (2007).
Harding, A. H. et al. Dietary fat and the risk of clinical type 2 diabetes: The European prospective investigation of Cancer-Norfolk study. Am. J. Epidemiol. 159, 73–82. https://doi.org/10.1093/aje/kwh004 (2004).
O’Mahoney, L. L. et al. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: A meta-analysis and meta-regression of randomized controlled trials. Cardiovasc. Diabetol. 17, 98. https://doi.org/10.1186/s12933-018-0740-x (2018).
Elajami, T. K. et al. Eicosapentaenoic and docosahexaenoic acids attenuate progression of albuminuria in patients with type 2 diabetes mellitus and coronary artery disease. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.116.004740 (2017).
Tao, M., McDowell, M. A., Saydah, S. H. & Eberhardt, M. S. Relationship of polyunsaturated fatty acid intake to peripheral neuropathy among adults with diabetes in the National Health and Nutrition Examination Survey (NHANES) 1999 2004. Diabetes Care 31, 93–95. https://doi.org/10.2337/dc07-0931 (2008).
Zhou, B. F. et al. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: The INTERMAP study. J. Hum. Hypertens. 17, 623–630. https://doi.org/10.1038/sj.jhh.1001605 (2003).
Iso, H. et al. Fat and protein intakes and risk of intraparenchymal hemorrhage among middle-aged Japanese. Am. J. Epidemiol. 157, 32–39 (2003).
Kodama, K. et al. Ethnic differences in the relationship between insulin sensitivity and insulin response: A systematic review and meta-analysis. Diabetes Care 36, 1789–1796. https://doi.org/10.2337/dc12-1235 (2013).
Sasaki, M. et al. The associations of dietary intake of polyunsaturated fatty acids with diabetic retinopathy in well-controlled diabetes. Invest. Ophthalmol. Vis. Sci. 56, 7473–7479. https://doi.org/10.1167/iovs.15-17485 (2015).
Sala-Vila, A. et al. Dietary marine omega-3 fatty acids and incident sight-threatening retinopathy in middle-aged and older individuals with type 2 diabetes: Prospective investigation from the PREDIMED trial. JAMA Ophthalmol. 134, 1142–1149. https://doi.org/10.1001/jamaophthalmol.2016.2906 (2016).
Alcubierre, N. et al. Association of low oleic acid intake with diabetic retinopathy in type 2 diabetic patients: A case-control study. Nutr. Metab. (Lond.) 13, 40. https://doi.org/10.1186/s12986-016-0099-5 (2016).
Houtsmuller, A. J., Zahn, K. J. & Henkes, H. E. Unsaturated fats and progression of diabetic retinopathy. Doc. Ophthalmol. 48, 363–371 (1980).
Howard-Williams, J. et al. Polyunsaturated fatty acids and diabetic retinopathy. Br. J. Ophthalmol. 69, 15–18 (1985).
Lyons, T. J. et al. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest. Ophthalmol. Vis. Sci. 45, 910–918. https://doi.org/10.1167/iovs.02-0648 (2004).
Keech, A. C. et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 370, 1687–1697. https://doi.org/10.1016/S0140-6736(07)61607-9 (2007).
Group, A. S. et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 363, 233–244. https://doi.org/10.1056/NEJMoa1001288 (2010).
Organization, W. H. Healthy diet. https://www.who.int/news-room/fact-sheets/detail/healthy-diet (2020).
Hooper, L. et al. Effects of total fat intake on body weight. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD011834 (2015).
Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser 916, i-viii, 1–149, backcover (2003).
Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food Nutr Pap 91, 1–166 (2010).
U.S.Department of Agriculture, Agricultural Research Service. Nutrient Intakes from Food and Beverages: Mean Amounts Consumed per Individual, by Gender and Age, What We Eat in America, NHANES 2017–2018, 2020).
Ministry of Health, Labour and Welfare, Japan.
Mozaffarian, D. & Rimm, E. B. Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 296, 1885–1899. https://doi.org/10.1001/jama.296.15.1885 (2006).
Yao, L. et al. The Triglyceride–Glucose index is associated with diabetic retinopathy in Chinese patients with type 2 diabetes: A hospital-based, nested, case-control study. Diabetes Metab. Syndr. Obes. 14, 1547–1555. https://doi.org/10.2147/DMSO.S294408 (2021).
Zhu, W., Meng, Y. F., Wu, Y., Xu, M. & Lu, J. Association of alcohol intake with risk of diabetic retinopathy: A meta-analysis of observational studies. Sci. Rep. 7, 4. https://doi.org/10.1038/s41598-017-00034-w (2017).
Dow, C. et al. Diet and risk of diabetic retinopathy: A systematic review. Eur. J. Epidemiol. 33, 141–156. https://doi.org/10.1007/s10654-017-0338-8 (2018).
Shah, J. et al. Dietary intake and diabetic retinopathy: A systematic review of the literature. Nutrients https://doi.org/10.3390/nu14235021 (2022).
Sawada, N. et al. The Japan Public Health Center-based Prospective Study for the Next Generation (JPHC-NEXT): Study design and participants. J. Epidemiol. 30, 46–54. https://doi.org/10.2188/jea.JE20180182 (2020).
Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes, M. et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J. Diabetes Investig. 1, 212–228. https://doi.org/10.1111/j.2040-1124.2010.00074.x (2010).
Teramoto, T. et al. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J. Atheroscler. Thromb. 14, 155–158 (2007).
Whitworth, J. A. & World Health Organization, I. S. o. H. W. G. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J. Hypertens. 21, 1983–1992. https://doi.org/10.1097/01.hjh.0000084751.37215.d2 (2003).
Yokoyama, Y. et al. Validity of short and long self-administered food frequency questionnaires in ranking dietary intake in middle-aged and elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 26, 420–432. https://doi.org/10.2188/jea.JE20150064 (2016).
Science and Technology Agency. Standard Tables of Food Composition in Japan. The Fifth Revised Edition (Printing Bureau, Ministry of Finance, Tokyo, 2000) (in Japanese).
Acknowledgements
We acknowledge the medical staff for their technical assistance and thank all staff members in Chikusei City for their extensive efforts in conducting the survey. We also thank Dr. Hidemasa Torii, Dr. Yusaku Katada, Dr. Erisa Yotsukura, Dr. Hiromitsu Kunimi, and Dr. Mari Ibuki of the Keio University School of Medicine for their cooperation in the diagnosis of retinal fundus images.
Funding
The authors have no proprietary or commercial interests in any material discussed in this article (financial or non-financial). This study was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI, 20K10490) to MS. The cohort study was originally supported by the National Cancer Center Research and Development Fund.
Author information
Authors and Affiliations
Contributions
Design of the study (M.S.); conduct of the study (M.S., K.Y., K.Y.); collection and management of data (M.S., K.Y., A.H., K.Y., K.M., T.K., Y.T., K.M., N.O., Y.O.); analysis of data (M.S.); interpretation of data (M.S., A.H., K.Y.); preparation of the manuscript (M.S.); review and approval of the manuscript (M.S., K.Y., A.H., K.Y., K.M., T.K., K.Y., N.O., Y.O., N.S., K.N., K.T., S.T., H.I.).
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sasaki, M., Yuki, K., Hanyuda, A. et al. Associations between fatty acid intake and diabetic retinopathy in a Japanese population. Sci Rep 13, 12903 (2023). https://doi.org/10.1038/s41598-023-39734-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-39734-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.