Abstract
The assessment of disease activity in systemic sclerosis (SSc) is challenging owing to its heterogeneous manifestations across multiple organ systems, the variable rate of disease progression and regression, and the relative paucity of patients in early-phase therapeutic trials. Despite some recent successes, most clinical trials have failed to show efficacy, underscoring the need for improved outcome measures linked directly to disease pathogenesis, particularly applicable for biomarker studies focused on skin disease. Current outcome measures in SSc-associated interstitial lung disease and SSc skin disease are largely adequate, although advancing imaging technology and the incorporation of skin mRNA biomarkers might provide opportunities for earlier detection of the therapeutic effect. Biomarkers can further inform pathogenesis, enabling early phase trials to act as reverse translational studies through the incorporation of routine high-throughput sequencing.
Key points
-
The majority of clinical trials for systemic sclerosis (SSc) focus on skin disease, interstitial lung disease (ILD), or pulmonary arterial hypertension, with modified Rodnan skin score (MRSS), forced vital capacity and 6-min walk distance being the primary clinical end points assessed, respectively.
-
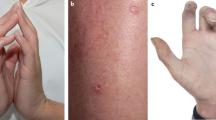
Increased variability in the natural history of SSc skin disease impedes the identification of treatment-related effects, and strategies for shorter duration trials might improve the separation of treatment effect from variation in disease natural history.
-
Forced vital capacity is an FDA-accepted surrogate outcome measure for SSc-ILD, whereas novel imaging technologies and serum biomarkers present opportunities for future additional SSc-ILD outcome measures to show significant changes at earlier timepoints.
-
Skin mRNA biomarkers, including THBS1 and COMP, could be considered as surrogate outcome measures in early-phase clinical trials, to more rapidly assess target engagement by therapeutic efficacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bournia, V. K. et al. All-cause mortality in systemic rheumatic diseases under treatment compared with the general population, 2015–2019. RMD Open https://doi.org/10.1136/rmdopen-2021-001694 (2021).
Mantero, J. C. et al. Randomised, double-blind, placebo-controlled trial of IL1-trap, rilonacept, in systemic sclerosis. A phase I/II biomarker trial. Clin. Exp. Rheumatol. 36 (Suppl. 113), 146–149 (2018).
Campochiaro, C. & Allanore, Y. An update on targeted therapies in systemic sclerosis based on a systematic review from the last 3 years. Arthritis Res. Ther. 23, 155 (2021).
Gordon, J. K. & Domsic, R. T. Clinical trial design issues in systemic sclerosis: an update. Curr. Rheumatol. Rep. 18, 38 (2016).
Denton, C. P. Challenges in systemic sclerosis trial design. Semin. Arthritis Rheum. 49, S3–S7 (2019).
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001).
Wipff, J. et al. Association of a KCNA5 gene polymorphism with systemic sclerosis-associated pulmonary arterial hypertension in the European Caucasian population. Arthritis Rheum. 62, 3093–3100 (2010).
Ouboussad, L., Burska, A. N., Melville, A. & Buch, M. H. Synovial tissue heterogeneity in rheumatoid arthritis and changes with biologic and targeted synthetic therapies to inform stratified therapy. Front. Med. 6, 45 (2019).
Humby, F. et al. Rituximab versus tocilizumab in anti-TNF inadequate responder patients with rheumatoid arthritis (R4RA): 16-week outcomes of a stratified, biopsy-driven, multicentre, open-label, phase 4 randomised controlled trial. Lancet 397, 305–317 (2021).
Merkel, P. A. et al. Patterns and predictors of change in outcome measures in clinical trials in scleroderma: an individual patient meta-analysis of 629 subjects with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 64, 3420–3429 (2012).
Domsic, R. T. Scleroderma: the role of serum autoantibodies in defining specific clinical phenotypes and organ system involvement. Curr. Opin. Rheumatol. 26, 646–652 (2014).
Pendergrass, S. A. et al. Intrinsic gene expression subsets of diffuse cutaneous systemic sclerosis are stable in serial skin biopsies. J. Invest. Dermatol. 132, 1363–1373 (2012).
Correia, C. et al. High-throughput quantitative histology in systemic sclerosis skin disease using computer vision. Arthritis Res. Ther. 22, 48 (2020).
Gabrielli, A., Avvedimento, E. V. & Krieg, T. Scleroderma. N. Engl. J. Med. 360, 1989–2003 (2009).
Lescoat, A. et al. Considerations for a combined index for limited cutaneous systemic sclerosis to support drug development and improve outcomes. J. Scleroderma Relat. Disord. 6, 66–76 (2021).
Ziemek, J. et al. The relationship between skin symptoms and the scleroderma modification of the health assessment questionnaire, the modified Rodnan skin score, and skin pathology in patients with systemic sclerosis. Rheumatology 55, 911–917 (2016).
Man, A. et al. Development and validation of a patient-reported outcome instrument for skin involvement in patients with systemic sclerosis. Ann. Rheum. Dis. 76, 1374–1380 (2017).
Matsuda, K. M. et al. Skin thickness score as a surrogate marker of organ involvements in systemic sclerosis: a retrospective observational study. Arthritis Res. Ther. 21, 129 (2019).
Kumanovics, G. et al. Assessment of skin involvement in systemic sclerosis. Rheumatology 56, v53–v66 (2017).
Khanna, D. et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2, 11–18 (2017).
van Laar, J. M. et al. Autologous hematopoietic stem cell transplantation vs intravenous pulse cyclophosphamide in diffuse cutaneous systemic sclerosis: a randomized clinical trial. JAMA 311, 2490–2498 (2014).
Clements, P. et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J. Rheumatol. 22, 1281–1285 (1995).
Naredo, E. et al. Performance of ultra-high-frequency ultrasound in the evaluation of skin involvement in systemic sclerosis: a preliminary report. Rheumatology 59, 1671–1678 (2020).
Santiago, T. et al. Ultrasonography for the assessment of skin in systemic sclerosis: a systematic review. Arthritis Care Res. 71, 563–574 (2019).
Sulli, A. et al. Subclinical dermal involvement is detectable by high frequency ultrasound even in patients with limited cutaneous systemic sclerosis. Arthritis Res. Ther. 19, 61 (2017).
Yang, Y. et al. Quantification of skin stiffness in patients with systemic sclerosis using real-time shear wave elastography: a preliminary study. Clin. Exp. Rheumatol. 36 (Suppl. 113), 118–125 (2018).
Kissin, E. Y. et al. Durometry for the assessment of skin disease in systemic sclerosis. Arthritis Rheum. 55, 603–609 (2006).
Merkel, P. A. et al. Validity, reliability, and feasibility of durometer measurements of scleroderma skin disease in a multicenter treatment trial. Arthritis Rheum. 59, 699–705 (2008).
Palamar, D. et al. Disease activity, handgrip strengths, and hand dexterity in patients with rheumatoid arthritis. Clin. Rheumatol. 36, 2201–2208 (2017).
Stoenoiu, M. S., Houssiau, F. A. & Lecouvet, F. E. Tendon friction rubs in systemic sclerosis: a possible explanation-an ultrasound and magnetic resonance imaging study. Rheumatology 52, 529–533 (2013).
Sandler, R. D., Matucci-Cerinic, M. & Hughes, M. Musculoskeletal hand involvement in systemic sclerosis. Semin. Arthritis Rheum. 50, 329–334 (2020).
Torok, K. S. et al. Reliability and validity of the delta finger-to-palm (FTP), a new measure of finger range of motion in systemic sclerosis. Clin. Exp. Rheumatol. 28, S28–S36 (2010).
Javinani, A. et al. The clinical value of the delta finger to palm distance in systemic sclerosis. Reumatismo 72, 44–51 (2020).
Sandqvist, G., Nilsson, J. A., Wuttge, D. M. & Hesselstrand, R. Development of a modified hand mobility in scleroderma (HAMIS) test and its potential as an outcome measure in systemic sclerosis. J. Rheumatol. 41, 2186–2192 (2014).
Sandqvist, G. & Eklund, M. Validity of HAMIS: a test of hand mobility in scleroderma. Arthritis Care Res. 13, 382–387 (2000).
Mittoo, S. et al. Patient perspectives in OMERACT provide an anchor for future metric development and improved approaches to healthcare delivery in connective tissue disease related interstitial lung disease (CTD-ILD). Curr. Respir. Med. Rev. 11, 175–183 (2015).
Saketkoo, L. A. et al. Reconciling healthcare professional and patient perspectives in the development of disease activity and response criteria in connective tissue disease-related interstitial lung diseases. J. Rheumatol. 41, 792–798 (2014).
Man, A., Dgetluck, N., Conley, B. & White, B. FRI0334 performance of the scleroderma skin patient-reported outcome (SSPRO) in a phase 2 trial with lenabasum. Ann. Rheum. Dis. 78 (Suppl. 2), 848.3–849 (2019).
Rannou, F. et al. Assessing disability and quality of life in systemic sclerosis: construct validities of the Cochin Hand Function Scale, Health Assessment Questionnaire (HAQ), Systemic Sclerosis HAQ, and medical outcomes study 36-item short form health survey. Arthritis Rheum. 57, 94–102 (2007).
Levis, A. W. et al. Using optimal test assembly methods for shortening patient-reported outcome measures: development and validation of the cochin hand function scale-6: a scleroderma patient-centered intervention network cohort study. Arthritis Care Res. 68, 1704–1713 (2016).
Schouffoer, A. A. et al. Validity and responsiveness of the Michigan Hand Questionnaire in patients with systemic sclerosis. Rheumatology 55, 1386–1393 (2016).
Mouthon, L. et al. Psychometric validation of the hand disability in systemic sclerosis-digital ulcers (HDISS-DU®) patient-reported outcome instrument. Arthritis Res. Ther. 22, 3 (2020).
Clements, P., Allanore, Y., Furst, D. E. & Khanna, D. Points to consider for designing trials in systemic sclerosis patients with arthritic involvement. Rheumatology 56, v23–v26 (2017).
Becker, M. O. et al. Development and validation of a patient-reported outcome measure for systemic sclerosis: the EULAR systemic sclerosis impact of disease (ScleroID) questionnaire. Ann. Rheum. Dis. 81, 507–515 (2022).
Benan, M., Hande, I. & Gul, O. The natural course of progressive systemic sclerosis patients with interstitial lung involvement. Clin. Rheumatol. 26, 349–354 (2007).
Steen, V. D. & Medsger, T. A. Changes in causes of death in systemic sclerosis, 1972–2002. Ann. Rheum. Dis. 66, 940–944 (2007).
Hoffmann-Vold, A. M. et al. Tracking impact of interstitial lung disease in systemic sclerosis in a complete nationwide cohort. Am. J. Respir. Crit. Care Med. 200, 1258–1266 (2019).
Distler, O. et al. Predictors of progression in systemic sclerosis patients with interstitial lung disease. Eur. Respir. J. https://doi.org/10.1183/13993003.02026-2019 (2020).
Khanna, D. & Merkel, P. A. Outcome measures in systemic sclerosis: an update on instruments and current research. Curr. Rheumatol. Rep. 9, 151–157 (2007).
Tashkin, D. P. et al. Cyclophosphamide versus placebo in scleroderma lung disease. N. Engl. J. Med. 354, 2655–2666 (2006).
Tashkin, D. P. et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir. Med. 4, 708–719 (2016).
Distler, O. et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N. Engl. J. Med. 380, 2518–2528 (2019).
Caron, M., Hoa, S., Hudson, M., Schwartzman, K. & Steele, R. Pulmonary function tests as outcomes for systemic sclerosis interstitial lung disease. Eur. Respir. Rev. https://doi.org/10.1183/16000617.0102-2017 (2018).
Suliman, Y. A. et al. Brief report: pulmonary function tests: high rate of false-negative results in the early detection and screening of scleroderma-related interstitial lung disease. Arthritis Rheumatol. 67, 3256–3261 (2015).
Wells, A. U. et al. Fibrosing alveolitis in systemic sclerosis: indices of lung function in relation to extent of disease on computed tomography. Arthritis Rheum. 40, 1229–1236 (1997).
Tashkin, D. P. et al. Relationship between quantitative radiographic assessments of interstitial lung disease and physiological and clinical features of systemic sclerosis. Ann. Rheum. Dis. 75, 374–381 (2016).
Wells, A. U., Behr, J. & Silver, R. Outcome measures in the lung. Rheumatology 47 (Suppl. 5), v48–v50 (2008).
Khanna, D. et al. Systemic sclerosis-associated interstitial lung disease: lessons from clinical trials, outcome measures, and future study design. Curr. Rheumatol. Rev. 6, 138–144 (2010).
Kafaja, S. et al. Reliability and minimal clinically important differences of forced vital capacity: results from the scleroderma lung studies (SLS-I and SLS-II). Am. J. Respir. Crit. Care Med. 197, 644–652 (2018).
Winstone, T. A. et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest 146, 422–436 (2014).
Moore, O. A. et al. Quantifying change in pulmonary function as a prognostic marker in systemic sclerosis-related interstitial lung disease. Clin. Exp. Rheumatol. 33, S111–S116 (2015).
Goh, N. S. et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol. 69, 1670–1678 (2017).
Khanna, D. et al. Connective tissue disease-associated interstitial lung diseases (CTD-ILD) — report from OMERACT CTD-ILD Working Group. J. Rheumatol. 42, 2168–2171 (2015).
Kowal-Bielecka, O. et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann. Rheum. Dis. 76, 1327–1339 (2017).
Hoffman-Vold, A. M. et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheum. 2, E71–E83 (2020).
Molberg, O. & Hoffmann-Vold, A. M. Interstitial lung disease in systemic sclerosis: progress in screening and early diagnosis. Curr. Opin. Rheumatol. 28, 613–618 (2016).
Mehrabi, S., Moradi, M. M., Khodamoradi, Z. & Nazarinia, M. A. Effects of N-acetylcysteine on pulmonary functions in patients with systemic sclerosis: a randomized double blind, placebo controlled study. Curr. Rheumatol. Rev. 16, 149–157 (2020).
Buch, M. H. et al. Submaximal exercise testing in the assessment of interstitial lung disease secondary to systemic sclerosis: reproducibility and correlations of the 6-min walk test. Ann. Rheum. Dis. 66, 169–173 (2007).
Schoindre, Y. et al. Lack of specificity of the 6-minute walk test as an outcome measure for patients with systemic sclerosis. J. Rheumatol. 36, 1481–1485 (2009).
Sanges, S. et al. A prospective study of the 6 min walk test as a surrogate marker for haemodynamics in two independent cohorts of treatment-naive systemic sclerosis-associated pulmonary arterial hypertension. Ann. Rheum. Dis. 75, 1457–1465 (2016).
Ewert, R. et al. Prognostic value of cardiopulmonary exercise testing in patients with systemic sclerosis. BMC Pulm. Med. 19, 230 (2019).
Hemelein, R. A. et al. Evaluation of cardiopulmonary exercise test in the prediction of disease progression in systemic sclerosis. Clin. Exp. Rheumatol. 39 (Suppl. 131), 94–102 (2021).
Khanna, D. et al. Tocilizumab in systemic sclerosis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 8, 963–974 (2020).
Burt, R. K. et al. Autologous non-myeloablative haemopoietic stem-cell transplantation compared with pulse cyclophosphamide once per month for systemic sclerosis (ASSIST): an open-label, randomised phase 2 trial. Lancet 378, 498–506 (2011).
Sperandeo, M. et al. Ultrasound signs of pulmonary fibrosis in systemic sclerosis as timely indicators for chest computed tomography. Scand. J. Rheumatol. 44, 389–398 (2015).
Tardella, M. et al. Ultrasound in the assessment of pulmonary fibrosis in connective tissue disorders: correlation with high-resolution computed tomography. J. Rheumatol. 39, 1641–1647 (2012).
Gutierrez, M. et al. Utility of a simplified ultrasound assessment to assess interstitial pulmonary fibrosis in connective tissue disorders-preliminary results. Arthritis Res. Ther. 13, R134 (2011).
Gutierrez, M. et al. Ultrasound in the assessment of interstitial lung disease in systemic sclerosis: a systematic literature review by the OMERACT Ultrasound Group. J. Rheumatol. 47, 991–1000 (2020).
Ledoult, E. et al. 18F-FDG positron emission tomography scanning in systemic sclerosis-associated interstitial lung disease: a pilot study. Arthritis Res. Ther. 23, 76 (2021).
Jacquelin, V. et al. FDG-PET/CT in the prediction of pulmonary function improvement in nonspecific interstitial pneumonia. A pilot study. Eur. J. Radiol. 85, 2200–2205 (2016).
Schniering, J. et al. Evaluation of 99mTc-rhAnnexin V-128 SPECT/CT as a diagnostic tool for early stages of interstitial lung disease associated with systemic sclerosis. Arthritis Res. Ther. 20, 183 (2018).
Schniering, J. et al. Visualisation of interstitial lung disease by molecular imaging of integrin αvβ3 and somatostatin receptor 2. Ann. Rheum. Dis. 78, 218–227 (2019).
Wallace, B. et al. Reliability, validity and responsiveness to change of the Saint George’s respiratory questionnaire in early diffuse cutaneous systemic sclerosis. Rheumatology 54, 1369–1379 (2015).
Hoffmann-Vold, A. M. & Molberg, O. Detection, screening, and classification of interstitial lung disease in patients with systemic sclerosis. Curr. Opin. Rheumatol. 32, 497–504 (2020).
Saketkoo, L. A., Scholand, M. B., Lammi, M. R. & Russell, A. M. Patient-reported outcome measures in systemic sclerosis-related interstitial lung disease for clinical practice and clinical trials. J. Scleroderma Relat. Disord. 5, 48–60 (2020).
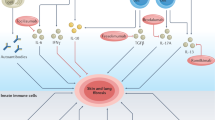
Farina, G., Lafyatis, D., Lemaire, R. & Lafyatis, R. A four-gene biomarker predicts skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheum. 62, 580–588 (2010).
Rice, L. M. et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J. Clin. Invest. 125, 2795–2807 (2015).
Khanna, D. et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet 387, 2630–2640 (2016).
Tabib, T. et al. Myofibroblast transcriptome indicates SFRP2hi fibroblast progenitors in systemic sclerosis skin. Nat. Commun. 12, 4384 (2021).
Tabib, T., Morse, C., Wang, T., Chen, W. & Lafyatis, R. SFRP2/DPP4 and FMO1/LSP1 define major fibroblast populations in human skin. J. Invest. Dermatol. 138, 802–810 (2018).
Xue, D. et al. Expansion of FCGR3A+ macrophages, FCN1+ mo-DC, and plasmacytoid dendritic cells associated with severe skin disease in systemic sclerosis. Arthritis Rheumatol. https://doi.org/10.1002/art.41813 (2021).
Stifano, G. et al. Skin gene expression is prognostic for the trajectory of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 70, 912–919 (2018).
Chen, G., Ning, B. & Shi, T. Single-cell RNA-Seq technologies and related computational data analysis. Front. Genet. 10, 317 (2019).
Rice, L. M. et al. A longitudinal biomarker for the extent of skin disease in patients with diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 67, 3004–3015 (2015).
Shirai, Y., Fukue, R., Kaneko, Y. & Kuwana, M. Clinical relevance of the serial measurement of Krebs von den Lungen — 6 levels in patients with systemic sclerosis-associated interstitial lung disease. Diagnostics https://doi.org/10.3390/diagnostics11112007 (2021).
Khanna, D. et al. Etiology, risk factors, and biomarkers in systemic sclerosis with interstitial lung disease. Am. J. Respir. Crit. Care Med. 201, 650–660 (2020).
Elhai, M. et al. Performance of candidate serum biomarkers for systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol. 71, 972–982 (2019).
Valenzi, E. et al. Single-cell analysis reveals fibroblast heterogeneity and myofibroblasts in systemic sclerosis-associated interstitial lung disease. Ann. Rheum. Dis. 78, 1379–1387 (2019).
Yamakawa, H. et al. Serum KL-6 and surfactant protein-D as monitoring and predictive markers of interstitial lung disease in patients with systemic sclerosis and mixed connective tissue disease. J. Thorac. Dis. 9, 362–371 (2017).
Volkmann, E. R. et al. Progression of interstitial lung disease in systemic sclerosis: the importance of pneumoproteins Krebs von den Lungen 6 and CCL18. Arthritis Rheumatol. 71, 2059–2067 (2019).
Greene, K. E. et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am. J. Respir. Crit. Care Med. 160, 1843–1850 (1999).
Yanaba, K., Hasegawa, M., Takehara, K. & Sato, S. Comparative study of serum surfactant protein-D and KL-6 concentrations in patients with systemic sclerosis as markers for monitoring the activity of pulmonary fibrosis. J. Rheumatol. 31, 1112–1120 (2004).
Sumida, H. et al. Prediction of therapeutic response before and during i.v. cyclophosphamide pulse therapy for interstitial lung disease in systemic sclerosis: a longitudinal observational study. J. Dermatol. 45, 1425–1433 (2018).
Abignano, G. & Del Galdo, F. Biomarkers as an opportunity to stratify for outcome in systemic sclerosis. Eur. J. Rheumatol. 7, S193–S202 (2020).
Tiev, K. P. et al. Serum CC chemokine ligand-18 predicts lung disease worsening in systemic sclerosis. Eur. Respir. J. 38, 1355–1360 (2011).
Kuwana, M., Shirai, Y. & Takeuchi, T. Elevated serum Krebs von den Lungen-6 in early disease predicts subsequent deterioration of pulmonary function in patients with systemic sclerosis and interstitial lung disease. J. Rheumatol. 43, 1825–1831 (2016).
Schupp, J. et al. Serum CCL18 is predictive for lung disease progression and mortality in systemic sclerosis. Eur. Respir. J. 43, 1530–1532 (2014).
Hoffmann-Vold, A. M. et al. High level of chemokine CCL18 is associated with pulmonary function deterioration, lung fibrosis progression, and reduced survival in systemic sclerosis. Chest 150, 299–306 (2016).
Fernando, M. R., Reyes, J. L., Iannuzzi, J., Leung, G. & McKay, D. M. The pro-inflammatory cytokine, interleukin-6, enhances the polarization of alternatively activated macrophages. PLoS One 9, e94188 (2014).
Lorenzen, J. M. et al. Osteopontin in the development of systemic sclerosis-relation to disease activity and organ manifestation. Rheumatology 49, 1989–1991 (2010).
Valenzi, E. et al. Disparate interferon signaling and shared aberrant basaloid cells in single-cell profiling of idiopathic pulmonary fibrosis and systemic sclerosis-associated interstitial lung disease. Front. Immunol. 12, 595811 (2021).
Morse, C. et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur. Respir. J. https://doi.org/10.1183/13993003.02441-2018 (2019).
Sproston, N. R. & Ashworth, J. J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 9, 754 (2018).
Liu, X. et al. Does C-reactive protein predict the long-term progression of interstitial lung disease and survival in patients with early systemic sclerosis? Arthritis Care Res. 65, 1375–1380 (2013).
Ross, L. et al. The role of inflammatory markers in assessment of disease activity in systemic sclerosis. Clin. Exp. Rheumatol. 36 (Suppl. 113), 126–134 (2018).
Chowaniec, M., Skoczynska, M., Sokolik, R. & Wiland, P. Interstitial lung disease in systemic sclerosis: challenges in early diagnosis and management. Reumatologia 56, 249–254 (2018).
Khanna, D. et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate). Ann. Rheum. Dis. 77, 212–220 (2018).
Ronnblom, L. & Alm, G. V. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J. Exp. Med. 194, F59–F63 (2001).
Farkas, L., Beiske, K., Lund-Johansen, F., Brandtzaeg, P. & Jahnsen, F. L. Plasmacytoid dendritic cells (natural interferon- α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159, 237–243 (2001).
van Bon, L. et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N. Engl. J. Med. 370, 433–443 (2014).
Kafaja, S. et al. pDCs in lung and skin fibrosis in a bleomycin-induced model and patients with systemic sclerosis. JCI Insight https://doi.org/10.1172/jci.insight.98380 (2018).
Volkmann, E. R. et al. Changes in plasma CXCL4 levels are associated with improvements in lung function in patients receiving immunosuppressive therapy for systemic sclerosis-related interstitial lung disease. Arthritis Res. Ther. 18, 305 (2016).
Lande, R. et al. CXCL4 assembles DNA into liquid crystalline complexes to amplify TLR9-mediated interferon-alpha production in systemic sclerosis. Nat. Commun. 10, 1731 (2019).
Guiot, J. et al. Serum IGFBP-2 in systemic sclerosis as a prognostic factor of lung dysfunction. Sci. Rep. 11, 10882 (2021).
Wu, M. et al. CCL2 in the circulation predicts long-term progression of interstitial lung disease in patients with early systemic sclerosis: data from two independent cohorts. Arthritis Rheumatol. 69, 1871–1878 (2017).
De Lauretis, A. et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J. Rheumatol. 40, 435–446 (2013).
Moinzadeh, P. et al. Elevated MMP-7 levels in patients with systemic sclerosis: correlation with pulmonary involvement. Exp. Dermatol. 20, 770–773 (2011).
Kim, W. U. et al. Elevated matrix metalloproteinase-9 in patients with systemic sclerosis. Arthritis Res. Ther. 7, R71–R79 (2005).
Abignano, G. et al. The enhanced liver fibrosis test: a clinical grade, validated serum test, biomarker of overall fibrosis in systemic sclerosis. Ann. Rheum. Dis. 73, 420–427 (2014).
Abignano, G. et al. European multicentre study validates enhanced liver fibrosis test as biomarker of fibrosis in systemic sclerosis. Rheumatology 58, 254–259 (2019).
Liu, X. et al. Correlation of interferon-inducible chemokine plasma levels with disease severity in systemic sclerosis. Arthritis Rheum. 65, 226–235 (2013).
Assassi, S. et al. Myeloablation followed by autologous stem cell transplantation normalises systemic sclerosis molecular signatures. Ann. Rheum. Dis. 78, 1371–1378 (2019).
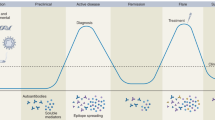
Dobrota, R. et al. Prediction of improvement in skin fibrosis in diffuse cutaneous systemic sclerosis: a EUSTAR analysis. Ann. Rheum. Dis. 75, 1743–1748 (2016).
Mihai, C., Dobrota, R., Assassi, S., Mayes, M. D. & Distler, O. Enrichment strategy for systemic sclerosis clinical trials targeting skin fibrosis: a prospective, multiethnic cohort study. ACR Open. Rheumatol. 2, 496–502 (2020).
Zhang, Y. & Michelakis, E. D. A phase-2 NIH-sponsored randomized clinical trial of rituximab in scleroderma-associated pulmonary arterial hypertension did not reach significance for its endpoints: end of story? Not so fast! Am. J. Respir. Crit. Care Med. 204, 123–125 (2021).
Khanna, D. et al. Abatacept in early diffuse cutaneous systemic sclerosis: results of a phase II investigator-initiated, multicenter, double-blind, randomized, placebo-controlled trial. Arthritis Rheumatol. 72, 125–136 (2020).
Zamanian, R. T. et al. Safety and efficacy of B-cell depletion with rituximab for the treatment of systemic sclerosis-associated pulmonary arterial hypertension: a multicenter, double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 204, 209–221 (2021).
Rubin, L. J. et al. Bosentan therapy for pulmonary arterial hypertension. N. Engl. J. Med. 346, 896–903 (2002).
Galie, N. et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N. Engl. J. Med. 353, 2148–2157 (2005).
Rovin, B. H. et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 397, 2070–2080 (2021).
Furie, R. et al. Two-year, randomized, controlled trial of Belimumab in lupus nephritis. N. Engl. J. Med. 383, 1117–1128 (2020).
Bombardier, C., Gladman, D. D., Urowitz, M. B., Caron, D. & Chang, C. H. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 35, 630–640 (1992).
Khanna, D. et al. Measures of response in clinical trials of systemic sclerosis: the combined response index for systemic sclerosis (CRISS) and outcome measures in pulmonary arterial hypertension related to systemic sclerosis (EPOSS). J. Rheumatol. 36, 2356–2361 (2009).
Valentini, G. et al. European Scleroderma Study Group to define disease activity criteria for systemic sclerosis. III. Assessment of the construct validity of the preliminary activity criteria. Ann. Rheum. Dis. 62, 901–903 (2003).
Valentini, G. et al. European multicentre study to define disease activity criteria for systemic sclerosis. II. Identification of disease activity variables and development of preliminary activity indexes. Ann. Rheum. Dis. 60, 592–598 (2001).
Groseanu, L. et al. Do we have good activity indices in systemic sclerosis? Curr. Rheumatol. Rev. https://doi.org/10.2174/1573397117666210913102759 (2021).
Nevskaya, T., Baron, M. & Pope, J. E., Canadian Scleroderma Research Group. Predictive value of European Scleroderma Group Activity Index in an early scleroderma cohort. Rheumatology 56, 1111–1122 (2017).
Ross, L. et al. Performance of the 2017 EUSTAR activity index in an scleroderma cohort. Clin. Rheumatol. 39, 3701–3705 (2020).
Valentini, G. et al. The European Scleroderma Trials and Research Group (EUSTAR) task force for the development of revised activity criteria for systemic sclerosis: derivation and validation of a preliminarily revised EUSTAR activity index. Ann. Rheum. Dis. 76, 270–276 (2017).
Fasano, S. et al. Revised European Scleroderma Trials and Research Group Activity Index is the best predictor of short-term severity accrual. Ann. Rheum. Dis. 78, 1681–1685 (2019).
Freemantle, N., Calvert, M., Wood, J., Eastaugh, J. & Griffin, C. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA 289, 2554–2559 (2003).
FDA. Surrogate endpoint resources for drug and biologic development. US Food & Drug Administration. https://www.fda.gov/drugs/development-resources/surrogate-endpoint-resources-drug-and-biologic-development (2018).
FDA. Table of surrogate endpoints that were the basis of drug approval or licensure. US Food & Drug Administration. https://www.fda.gov/drugs/development-resources/table-surrogate-endpoints-were-basis-drug-approval-or-licensure (2022).
Sumpton, D. et al. Scope and consistency of outcomes reported in trials of patients with systemic sclerosis. Arthritis Care Res. 72, 1449–1458 (2020).
Pulido, T. et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N. Engl. J. Med. 369, 809–818 (2013).
Khanna, D. et al. Reliability and validity of the University of California, Los Angeles Scleroderma Clinical Trial Consortium Gastrointestinal Tract Instrument. Arthritis Rheum. 61, 1257–1263 (2009).
Low, A. H. L. et al. A double-blind randomized placebo-controlled trial of probiotics in systemic sclerosis associated gastrointestinal disease. Semin. Arthritis Rheum. 49, 411–419 (2019).
Zampatti, N. et al. Performance of the UCLA Scleroderma clinical trials consortium gastrointestinal tract 2.0 instrument as a clinical decision aid in the routine clinical care of patients with systemic sclerosis. Arthritis Res. Ther. 23, 125 (2021).
Thurm, R. H. & Alexander, J. C. Captopril in the treatment of scleroderma renal crisis. Arch. Intern. Med. 144, 733–735 (1984).
Valentini, G. et al. Vasodilators and low-dose acetylsalicylic acid are associated with a lower incidence of distinct primary myocardial disease manifestations in systemic sclerosis: results of the DeSScipher inception cohort study. Ann. Rheum. Dis. 78, 1576–1582 (2019).
Daoussis, D., Antonopoulos, I., Liossis, S. N., Yiannopoulos, G. & Andonopoulos, A. P. Treatment of systemic sclerosis-associated calcinosis: a case report of rituximab-induced regression of CREST-related calcinosis and review of the literature. Semin. Arthritis Rheum. 41, 822–829 (2012).
Reiter, N., El-Shabrawi, L., Leinweber, B., Berghold, A. & Aberer, E. Calcinosis cutis: part II. Treatment options. J. Am. Acad. Dermatol. 65, 15–22; quiz 23–24 (2011).
Chung, L. et al. Validation of a novel radiographic scoring system for calcinosis affecting the hands of patients with systemic sclerosis. Arthritis Care Res. 67, 425–430 (2015).
Jones, D. K., Higenbottam, T. W. & Wallwork, J. Treatment of primary pulmonary hypertension intravenous epoprostenol (prostacyclin). Br. Heart J. 57, 270–278 (1987).
Williamson, D. J. et al. Hemodynamic effects of bosentan, an endothelin receptor antagonist, in patients with pulmonary hypertension. Circulation 102, 411–418 (2000).
Bhatia, S., Frantz, R. P., Severson, C. J., Durst, L. A. & McGoon, M. D. Immediate and long-term hemodynamic and clinical effects of sildenafil in patients with pulmonary arterial hypertension receiving vasodilator therapy. Mayo Clin. Proc. 78, 1207–1213 (2003).
Hsu, V., Varga, J. & Schlesinger, N. Calcinosis in scleroderma made crystal clear. Curr. Opin. Rheumatol. 31, 589–594 (2019).
Pokeerbux, M. R., Farhat, M. M., Merger, M., Launay, D. & Hachulla, E. Calcinosis in systemic sclerosis. Jt. Bone Spine 88, 105180 (2021).
Roofeh, D. et al. Outcome measurement instrument selection for lung physiology in systemic sclerosis associated interstitial lung disease: a systematic review using the OMERACT filter 2.1 process. Semin. Arthritis Rheum. 51, 1331–1341 (2021).
Philippeos, C. et al. Spatial and single-cell transcriptional profiling identifies functionally distinct human dermal fibroblast subpopulations. J. Invest. Dermatol. 138, 811–825 (2018).
Acknowledgements
R.L. is supported by the National Institutes of Health. E.V. is supported by the National Scleroderma Foundation and the Pulmonary Fibrosis Foundation.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
R.L. has served as a consultant for Pfizer, Bristol Myers Squibb, Boehringer-Ingelheim, Formation, Sanofi, Boehringer-Mannheim, Merck and Genentech/Roche, and holds or recently had research grants from Corbus, Formation, Moderna, Regeneron, Pfizer, and Kiniksa. E.V. declares no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lafyatis, R., Valenzi, E. Assessment of disease outcome measures in systemic sclerosis. Nat Rev Rheumatol 18, 527–541 (2022). https://doi.org/10.1038/s41584-022-00803-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00803-6
This article is cited by
-
Immune profiling analysis of double-negative T cells in patients with systemic sclerosis
Clinical Rheumatology (2024)
-
Low cognitive functioning and depressive symptoms in patients with rheumatoid arthritis and systemic sclerosis: a clinical study
BMC Psychiatry (2023)
-
The role of immunosuppressive myofibroblasts in the aging process and age-related diseases
Journal of Molecular Medicine (2023)
-
Antibody-mediated neutralization of galectin-3 as a strategy for the treatment of systemic sclerosis
Nature Communications (2023)