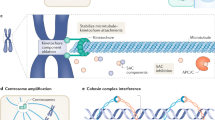
Abstract
Aneuploidy, a genomic alternation characterized by deviations in the copy number of chromosomes, affects organisms from early development through to aging. Although it is a main cause of human pregnancy loss and a hallmark of cancer, how aneuploidy affects cellular function has been elusive. The last two decades have seen rapid advances in the understanding of the causes and consequences of aneuploidy at the molecular and cellular levels. These studies have uncovered effects of aneuploidy that can be beneficial or detrimental to cells and organisms in an environmental context-dependent and karyotype-dependent manner. Aneuploidy also imposes general stress on cells that stems from an imbalanced genome and, consequently, also an imbalanced proteome. These insights provide the fundamental framework for understanding the impact of aneuploidy in genome evolution, human pathogenesis and drug resistance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tackholm, G. Zytologische Studien uber die Gattung Rosa. Acta Hort. Bergiani 7, 97–381 (1922).
Edwards, J. H., Harnden, D. G., Cameron, A. H., Crosse, V. M. & Wolff, O. H. A new trisomic syndrome. Lancet 1, 787–790 (1960).
Lejeune, J., Gautier, M. & Turpin, R. Study of somatic chromosomes from 9 mongoloid children. C. R. Hebd. Seances Acad. Sci. 248, 1721–1722 (1959).
Patau, K., Smith, D. W., Therman, E., Inhorn, S. L. & Wagner, H. P. Multiple congenital anomaly caused by an extra autosome. Lancet 1, 790–793 (1960).
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010).
Taylor, A. M. et al. Genomic and functional approaches to understanding cancer aneuploidy. Cancer Cell 33, 676–689.e3 (2018).
Khush, G. Cytogenetics of Aneuploids (Academic, 1973).
Gilchrist, C. & Stelkens, R. Aneuploidy in yeast: segregation error or adaptation mechanism? Yeast 36, 525–539 (2019).
Duijf, P. H., Schultz, N. & Benezra, R. Cancer cells preferentially lose small chromosomes. Int. J. Cancer 132, 2316–2326 (2013).
Terao, C. et al. Chromosomal alterations among age-related haematopoietic clones in Japan. Nature 584, 130–135 (2020).
McConnell, M. J. et al. Mosaic copy number variation in human neurons. Science 342, 632–637 (2013).
Duncan, A. W. et al. Frequent aneuploidy among normal human hepatocytes. Gastroenterology 142, 25–28 (2012).
Knouse, K. A., Wu, J., Whittaker, C. A. & Amon, A. Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc. Natl Acad. Sci. USA 111, 13409–13414 (2014).
van den Bos, H. et al. Single-cell whole genome sequencing reveals no evidence for common aneuploidy in normal and Alzheimer’s disease neurons. Genome Biol. 17, 116 (2016).
Coorens, T. H. H. et al. Inherent mosaicism and extensive mutation of human placentas. Nature 592, 80–85 (2021).
McCoy, R. C. Mosaicism in preimplantation human embryos: when chromosomal abnormalities are the norm. Trends Genet. 33, 448–463 (2017).
Ricke, R. M. & van Deursen, J. M. Aneuploidy in health, disease, and aging. J. Cell Biol. 201, 11–21 (2013).
Stirling, P. C. et al. The complete spectrum of yeast chromosome instability genes identifies candidate CIN cancer genes and functional roles for ASTRA complex components. PLoS Genet. 7, e1002057 (2011).
Neumann, B. et al. Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature 464, 721–727 (2010).
Worrall, J. T. et al. Non-random mis-segregation of human chromosomes. Cell Rep. 23, 3366–3380 (2018).
Dumont, M. et al. Human chromosome-specific aneuploidy is influenced by DNA-dependent centromeric features. EMBO J. 39, e102924 (2020).
Gregan, J., Polakova, S., Zhang, L., Tolić-Nørrelykke, I. M. & Cimini, D. Merotelic kinetochore attachment: causes and effects. Trends Cell Biol. 21, 374–381 (2011).
Musacchio, A. & Salmon, E. D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8, 379–393 (2007).
Hanks, S. et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 36, 1159–1161 (2004).
Yost, S. et al. Biallelic TRIP13 mutations predispose to Wilms tumor and chromosome missegregation. Nat. Genet. 49, 1148–1151 (2017).
Snape, K. et al. Mutations in CEP57 cause mosaic variegated aneuploidy syndrome. Nat. Genet. 43, 527–529 (2011).
Vitre, B. D. & Cleveland, D. W. Centrosomes, chromosome instability (CIN) and aneuploidy. Curr. Opin. Cell Biol. 24, 809–815 (2012).
Watanabe, K., Takao, D., Ito, K. K., Takahashi, M. & Kitagawa, D. The Cep57-pericentrin module organizes PCM expansion and centriole engagement. Nat. Commun. 10, 931 (2019).
Chiang, T., Duncan, F. E., Schindler, K., Schultz, R. M. & Lampson, M. A. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr. Biol. 20, 1522–1528 (2010).
Lister, L. M. et al. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr. Biol. 20, 1511–1521 (2010).
Burkhardt, S. et al. Chromosome cohesion established by rec8-cohesin in fetal oocytes is maintained without detectable turnover in oocytes arrested for months in mice. Curr. Biol. 26, 678–685 (2016).
Thompson, D. J. et al. Genetic predisposition to mosaic Y chromosome loss in blood. Nature 575, 652–657 (2019).
Burrell, R. A. et al. Replication stress links structural and numerical cancer chromosomal instability. Nature 494, 492–496 (2013).
Zeman, M. K. & Cimprich, K. A. Causes and consequences of replication stress. Nat. Cell Biol. 16, 2–9 (2014).
Maya-Mendoza, A. et al. High speed of fork progression induces DNA replication stress and genomic instability. Nature 559, 279–284 (2018).
García-Muse, T. & Aguilera, A. Transcription-replication conflicts: how they occur and how they are resolved. Nat. Rev. Mol. Cell Biol. 17, 553–563 (2016).
Marchal, C., Sima, J. & Gilbert, D. M. Control of DNA replication timing in the 3D genome. Nat. Rev. Mol. Cell Biol. 20, 721–737 (2019).
Kotsantis, P., Petermann, E. & Boulton, S. J. Mechanisms of oncogene-induced replication stress: jigsaw falling into place. Cancer Discov. 8, 537–555 (2018).
Chan, K. L., Palmai-Pallag, T., Ying, S. & Hickson, I. D. Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat. Cell Biol. 11, 753–760 (2009).
Naim, V. & Rosselli, F. The FANC pathway and BLM collaborate during mitosis to prevent micro-nucleation and chromosome abnormalities. Nat. Cell Biol. 11, 761–768 (2009).
Chan, Y. W., Fugger, K. & West, S. C. Unresolved recombination intermediates lead to ultra-fine anaphase bridges, chromosome breaks and aberrations. Nat. Cell Biol. 20, 92–103 (2018).
Ait Saada, A. et al. Unprotected replication forks are converted into mitotic sister chromatid bridges. Mol. Cell 66, 398–410.e394 (2017).
Böhly, N., Kistner, M. & Bastians, H. Mild replication stress causes aneuploidy by deregulating microtubule dynamics in mitosis. Cell Cycle 18, 2770–2783 (2019).
Wilhelm, T. et al. Mild replication stress causes chromosome mis-segregation via premature centriole disengagement. Nat. Commun. 10, 3585 (2019).
Pardo, B., Crabbé, L. & Pasero, P. Signaling pathways of replication stress in yeast. FEMS Yeast. Res. 17, fow101 (2017).
Kabeche, L., Nguyen, H. D., Buisson, R. & Zou, L. A mitosis-specific and R loop-driven ATR pathway promotes faithful chromosome segregation. Science 359, 108–114 (2018).
Bakhoum, S. F., Kabeche, L., Murnane, J. P., Zaki, B. I. & Compton, D. A. DNA-damage response during mitosis induces whole-chromosome missegregation. Cancer Discov. 4, 1281–1289 (2014).
Hassold, T. & Hunt, P. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2, 280–291 (2001).
Perkins, A. T., Das, T. M., Panzera, L. C. & Bickel, S. E. Oxidative stress in oocytes during midprophase induces premature loss of cohesion and chromosome segregation errors. Proc. Natl Acad. Sci. USA 113, E6823–E6830 (2016).
Zuelke, K. A., Jones, D. P. & Perreault, S. D. Glutathione oxidation is associated with altered microtubule function and disrupted fertilization in mature hamster oocytes. Biol. Reprod. 57, 1413–1419 (1997).
Mihalas, B. P., De Iuliis, G. N., Redgrove, K. A., McLaughlin, E. A. & Nixon, B. The lipid peroxidation product 4-hydroxynonenal contributes to oxidative stress-mediated deterioration of the ageing oocyte. Sci. Rep. 7, 6247 (2017).
Lim, D. C. et al. Redox priming promotes Aurora A activation during mitosis. Sci. Signal. 13, eabb6707 (2020).
Wang, G. F. et al. Oxidative stress induces mitotic arrest by inhibiting Aurora A-involved mitotic spindle formation. Free Radic. Biol. Med. 103, 177–187 (2017).
Técher, H., Koundrioukoff, S., Nicolas, A. & Debatisse, M. The impact of replication stress on replication dynamics and DNA damage in vertebrate cells. Nat. Rev. Genet. 18, 535–550 (2017).
Chen, G., Bradford, W. D., Seidel, C. W. & Li, R. Hsp90 stress potentiates rapid cellular adaptation through induction of aneuploidy. Nature 482, 246–250 (2012).
Niikura, Y. et al. 17-AAG, an Hsp90 inhibitor, causes kinetochore defects: a novel mechanism by which 17-AAG inhibits cell proliferation. Oncogene 25, 4133–4146 (2006).
Tan, Z. et al. Environmental stresses induce karyotypic instability in colorectal cancer cells. Mol. Biol. Cell 30, 42–55 (2019).
Lancaster, O. M. et al. Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation. Dev. Cell 25, 270–283 (2013).
Knouse, K. A., Lopez, K. E., Bachofner, M. & Amon, A. Chromosome segregation fidelity in epithelia requires tissue architecture. Cell 175, 200–211.e13 (2018).
Denais, C. M. et al. Nuclear envelope rupture and repair during cancer cell migration. Science 352, 353–358 (2016).
Raab, M. et al. ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science 352, 359–362 (2016).
Irianto, J. et al. DNA damage follows repair factor depletion and portends genome variation in cancer cells after pore migration. Curr. Biol. 27, 210–223 (2017).
Birchler, J. A. & Veitia, R. A. Gene balance hypothesis: connecting issues of dosage sensitivity across biological disciplines. Proc. Natl Acad. Sci. USA 109, 14746–14753 (2012).
Hughes, T. R. et al. Widespread aneuploidy revealed by DNA microarray expression profiling. Nat. Genet. 25, 333–337 (2000).
Puddu, F. et al. Genome architecture and stability in the Saccharomyces cerevisiae knockout collection. Nature 573, 416–420 (2019).
Torres, E. M. et al. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science 317, 916–924 (2007).
Hou, J. et al. Global impacts of chromosomal imbalance on gene expression in Arabidopsis and other taxa. Proc. Natl Acad. Sci. USA 115, E11321–E11330 (2018).
Rancati, G. et al. Aneuploidy underlies rapid adaptive evolution of yeast cells deprived of a conserved cytokinesis motor. Cell 135, 879–893 (2008).
Hwang, S. et al. Consequences of aneuploidy in human fibroblasts with trisomy 21. Proc. Natl Acad. Sci. USA 118, e2014723118 (2021).
Sullivan, K. D. et al. Trisomy 21 consistently activates the interferon response. eLife 5, e16220 (2016).
Ahlfors, H. et al. Gene expression dysregulation domains are not a specific feature of Down syndrome. Nat. Commun. 10, 2489 (2019).
Stingele, S. et al. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol. Syst. Biol. 8, 608 (2012).
Guo, M. & Birchler, J. A. Trans-acting dosage effects on the expression of model gene systems in maize aneuploids. Science 266, 1999–2002 (1994).
Mellman, W. J., Oski, F. A., Tedesco, T. A., Maciera-Coelho, A. & Harris, H. Leucocyte enzymes in down’s syndrome. Lancet 2, 674–675 (1964).
Ried, T. et al. The consequences of chromosomal aneuploidy on the transcriptome of cancer cells. Biochim. Biophys. Acta 1819, 784–793 (2012).
Samata, M. & Akhtar, A. Dosage compensation of the X chromosome: a complex epigenetic assignment involving chromatin regulators and long noncoding RNAs. Annu. Rev. Biochem. 87, 323–350 (2018).
Hose, J. et al. Dosage compensation can buffer copy-number variation in wild yeast. eLife 4, e05462 (2015).
Zhang, Y. et al. Expression in aneuploid Drosophila S2 cells. PLoS Biol. 8, e1000320 (2010).
Zhang, A. et al. Global analysis of gene expression in response to whole-chromosome aneuploidy in hexaploid wheat. Plant. Physiol. 175, 828–847 (2017).
Makarevitch, I. & Harris, C. Aneuploidy causes tissue-specific qualitative changes in global gene expression patterns in maize. Plant. Physiol. 152, 927–938 (2010).
Aït Yahya-Graison, E. et al. Classification of human chromosome 21 gene-expression variations in Down syndrome: impact on disease phenotypes. Am. J. Hum. Genet. 81, 475–491 (2007).
Roy, B. et al. Autoregulation of yeast ribosomal proteins discovered by efficient search for feedback regulation. Commun. Biol. 3, 761 (2020).
Kemeny, S. et al. Spatial organization of chromosome territories in the interphase nucleus of trisomy 21 cells. Chromosoma 127, 247–259 (2018).
Hervé, B. et al. Aneuploidy: the impact of chromosome imbalance on nuclear organization and overall genome expression. Clin. Genet. 90, 35–48 (2016).
Braun, R. et al. Single chromosome aneuploidy induces genome-wide perturbation of nuclear organization and gene expression. Neoplasia 21, 401–412 (2019).
Brás, A., Rodrigues, A. S., Gomes, B. & Rueff, J. Down syndrome and microRNAs. Biomed. Rep. 8, 11–16 (2018).
Yin, Q. et al. MicroRNA-155 is an Epstein-Barr virus-induced gene that modulates Epstein-Barr virus-regulated gene expression pathways. J. Virol. 82, 5295–5306 (2008).
Pavelka, N. et al. Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature 468, 321–325 (2010).
Dephoure, N. et al. Quantitative proteomic analysis reveals posttranslational responses to aneuploidy in yeast. eLife 3, e03023 (2014).
Liu, Y., Beyer, A. & Aebersold, R. On the dependency of cellular protein levels on mRNA Abundance. Cell 165, 535–550 (2016).
Gonçalves, E. et al. Widespread post-transcriptional attenuation of genomic copy-number variation in cancer. Cell Syst. 5, 386–398.e4 (2017).
Liu, Y. et al. Systematic proteome and proteostasis profiling in human trisomy 21 fibroblast cells. Nat. Commun. 8, 1212 (2017).
Brennan, C. M. et al. Protein aggregation mediates stoichiometry of protein complexes in aneuploid cells. Genes Dev. 33, 1031–1047 (2019).
Muskens, I. S. et al. The genome-wide impact of trisomy 21 on DNA methylation and its implications for hematopoiesis. Nat. Commun. 12, 821 (2021).
Lu, J. et al. Global hypermethylation in fetal cortex of Down syndrome due to DNMT3L overexpression. Hum. Mol. Genet. 25, 1714–1727 (2016).
Gao, L. et al. Heritable alteration of DNA methylation induced by whole-chromosome aneuploidy in wheat. N. Phytol. 209, 364–375 (2016).
Lane, A. A. et al. Triplication of a 21q22 region contributes to B cell transformation through HMGN1 overexpression and loss of histone H3 Lys27 trimethylation. Nat. Genet. 46, 618–623 (2014).
Mowery, C. T. et al. Trisomy of a down syndrome critical region globally amplifies transcription via HMGN1 overexpression. Cell Rep. 25, 1898–1911.e5 (2018).
Mulla, W. A. et al. Aneuploidy as a cause of impaired chromatin silencing and mating-type specification in budding yeast. eLife 6, e27991 (2017).
Selmecki, A., Forche, A. & Berman, J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Science 313, 367–370 (2006).
Selmecki, A., Gerami-Nejad, M., Paulson, C., Forche, A. & Berman, J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol. Microbiol. 68, 624–641 (2008).
Yona, A. H. et al. Chromosomal duplication is a transient evolutionary solution to stress. Proc. Natl Acad. Sci. USA 109, 21010–21015 (2012).
Tan, Z. et al. Aneuploidy underlies a multicellular phenotypic switch. Proc. Natl Acad. Sci. USA 110, 12367–12372 (2013).
Antonarakis, S. E. et al. Down syndrome. Nat. Rev. Dis. Primers 6, 9 (2020).
Antonarakis, S. E. Down syndrome and the complexity of genome dosage imbalance. Nat. Rev. Genet. 18, 147–163 (2017).
Ahn, K. J. et al. DYRK1A BAC transgenic mice show altered synaptic plasticity with learning and memory defects. Neurobiol. Dis. 22, 463–472 (2006).
Hibaoui, Y. et al. Modelling and rescuing neurodevelopmental defect of Down syndrome using induced pluripotent stem cells from monozygotic twins discordant for trisomy 21. EMBO Mol. Med. 6, 259–277 (2014).
Nakano-Kobayashi, A. et al. Prenatal neurogenesis induction therapy normalizes brain structure and function in Down syndrome mice. Proc. Natl Acad. Sci. USA 114, 10268–10273 (2017).
Souchet, B. et al. Excitation/inhibition balance and learning are modified by Dyrk1a gene dosage. Neurobiol. Dis. 69, 65–75 (2014).
Chen, G. et al. Targeting the adaptability of heterogeneous aneuploids. Cell 160, 771–784 (2015).
Kucharavy, A., Rubinstein, B., Zhu, J. & Li, R. Robustness and evolvability of heterogeneous cell populations. Mol. Biol. Cell 29, 1400–1409 (2018).
Kaya, A. et al. Molecular signatures of aneuploidy-driven adaptive evolution. Nat. Commun. 11, 588 (2020).
Williams, B. R. et al. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science 322, 703–709 (2008).
Sheltzer, J. M., Torres, E. M., Dunham, M. J. & Amon, A. Transcriptional consequences of aneuploidy. Proc. Natl Acad. Sci. USA 109, 12644–12649 (2012).
Cromie, G. A., Tan, Z., Hays, M., Jeffery, E. W. & Dudley, A. M. Dissecting gene expression changes accompanying a ploidy-based phenotypic switch. G3 7, 233–246 (2017).
O’Duibhir, E. et al. Cell cycle population effects in perturbation studies. Mol. Syst. Biol. 10, 732 (2014).
Brauer, M. J. et al. Coordination of growth rate, cell cycle, stress response, and metabolic activity in yeast. Mol. Biol. Cell 19, 352–367 (2008).
Terhorst, A. et al. The environmental stress response causes ribosome loss in aneuploid yeast cells. Proc. Natl Acad. Sci. USA 117, 17031–17040 (2020).
Ho, Y. H., Shishkova, E., Hose, J., Coon, J. J. & Gasch, A. P. Decoupling yeast cell division and stress defense implicates mRNA repression in translational reallocation during stress. Curr. Biol. 28, 2673–2680.e4 (2018).
Ye, C. J., Regan, S., Liu, G., Alemara, S. & Heng, H. H. Understanding aneuploidy in cancer through the lens of system inheritance, fuzzy inheritance and emergence of new genome systems. Mol. Cytogenet. 11, 31 (2018).
Papp, I. et al. Structural instability of a transgene locus in tobacco is associated with aneuploidy. Plant. J. 10, 469–478 (1996).
Sheltzer, J. M. et al. Aneuploidy drives genomic instability in yeast. Science 333, 1026–1030 (2011).
Tijhuis, A. E., Johnson, S. C. & McClelland, S. E. The emerging links between chromosomal instability (CIN), metastasis, inflammation and tumour immunity. Mol. Cytogenet. 12, 17 (2019).
Pfister, K. et al. Identification of drivers of aneuploidy in breast tumors. Cell Rep. 23, 2758–2769 (2018).
Zhu, J., Pavelka, N., Bradford, W. D., Rancati, G. & Li, R. Karyotypic determinants of chromosome instability in aneuploid budding yeast. PLoS Genet. 8, e1002719 (2012).
Ryan, S. D. et al. Up-regulation of the mitotic checkpoint component Mad1 causes chromosomal instability and resistance to microtubule poisons. Proc. Natl Acad. Sci. USA 109, E2205–E2214 (2012).
Barnhart, E. L., Dorer, R. K., Murray, A. W. & Schuyler, S. C. Reduced Mad2 expression keeps relaxed kinetochores from arresting budding yeast in mitosis. Mol. Biol. Cell 22, 2448–2457 (2011).
Ohashi, A. et al. Aneuploidy generates proteotoxic stress and DNA damage concurrently with p53-mediated post-mitotic apoptosis in SAC-impaired cells. Nat. Commun. 6, 7668 (2015).
Crasta, K. et al. DNA breaks and chromosome pulverization from errors in mitosis. Nature 482, 53–58 (2012).
Zhang, C. Z. et al. Chromothripsis from DNA damage in micronuclei. Nature 522, 179–184 (2015).
Torres, E. M. et al. Identification of aneuploidy-tolerating mutations. Cell 143, 71–83 (2010).
Oromendia, A. B., Dodgson, S. E. & Amon, A. Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 26, 2696–2708 (2012).
Beaupere, C. et al. Genetic screen identifies adaptive aneuploidy as a key mediator of ER stress resistance in yeast. Proc. Natl Acad. Sci. USA 115, 9586–9591 (2018).
Larrimore, K. E., Barattin-Voynova, N. S., Reid, D. W. & Ng, D. T. W. Aneuploidy-induced proteotoxic stress can be effectively tolerated without dosage compensation, genetic mutations, or stress responses. BMC Biol. 18, 117 (2020).
Santaguida, S., Vasile, E., White, E. & Amon, A. Aneuploidy-induced cellular stresses limit autophagic degradation. Genes Dev. 29, 2010–2021 (2015).
Ariyoshi, K. et al. Induction of genomic instability and activation of autophagy in artificial human aneuploid cells. Mutat. Res. 790, 19–30 (2016).
Singla, S., Iwamoto-Stohl, L. K., Zhu, M. & Zernicka-Goetz, M. Autophagy-mediated apoptosis eliminates aneuploid cells in a mouse model of chromosome mosaicism. Nat. Commun. 11, 2958 (2020).
Donnelly, N., Passerini, V., Dürrbaum, M., Stingele, S. & Storchová, Z. HSF1 deficiency and impaired HSP90-dependent protein folding are hallmarks of aneuploid human cells. EMBO J. 33, 2374–2387 (2014).
Pogribna, M. et al. Homocysteine metabolism in children with Down syndrome: in vitro modulation. Am. J. Hum. Genet. 69, 88–95 (2001).
Caracausi, M. et al. Plasma and urinary metabolomic profiles of Down syndrome correlate with alteration of mitochondrial metabolism. Sci. Rep. 8, 2977 (2018).
Panagaki, T., Randi, E. B., Augsburger, F. & Szabo, C. Overproduction of H2S, generated by CBS, inhibits mitochondrial complex IV and suppresses oxidative phosphorylation in Down syndrome. Proc. Natl Acad. Sci. USA 116, 18769–18771 (2019).
Valenti, D., Manente, G. A., Moro, L., Marra, E. & Vacca, R. A. Deficit of complex I activity in human skin fibroblasts with chromosome 21 trisomy and overproduction of reactive oxygen species by mitochondria: involvement of the cAMP/PKA signalling pathway. Biochem. J. 435, 679–688 (2011).
Li, M. et al. The ATM-p53 pathway suppresses aneuploidy-induced tumorigenesis. Proc. Natl Acad. Sci. USA 107, 14188–14193 (2010).
Clemente-Ruiz, M. et al. Gene dosage imbalance contributes to chromosomal instability-induced tumorigenesis. Dev. Cell 36, 290–302 (2016).
Hwang, S. et al. Serine-dependent sphingolipid synthesis is a metabolic liability of aneuploid cells. Cell Rep. 21, 3807–3818 (2017).
Tang, Y. C. et al. Aneuploid cell survival relies upon sphingolipid homeostasis. Cancer Res. 77, 5272–5286 (2017).
Tsai, H. J. et al. Hypo-osmotic-like stress underlies general cellular defects of aneuploidy. Nature 570, 117–121 (2019).
Marx, J. Debate surges over the origins of genomic defects in cancer. Science 297, 544–546 (2002).
Dey, P. Aneuploidy and malignancy: an unsolved equation. J. Clin. Pathol. 57, 1245–1249 (2004).
Vasudevan, A. et al. Aneuploidy as a promoter and suppressor of malignant growth. Nat. Rev. Cancer 21, 89–103 (2021).
Ben-David, U. & Amon, A. Context is everything: aneuploidy in cancer. Nat. Rev. Genet. 21, 44–62 (2020).
Bakhoum, S. F. & Cantley, L. C. The multifaceted role of chromosomal instability in cancer and its microenvironment. Cell 174, 1347–1360 (2018).
Dimaras, H. et al. Retinoblastoma. Nat. Rev. Dis. Primers 1, 15021 (2015).
Cavenee, W. K. et al. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. Nature 305, 779–784 (1983).
Nowak, M. A. et al. The role of chromosomal instability in tumor initiation. Proc. Natl Acad. Sci. USA 99, 16226–16231 (2002).
Nichols, C. A. et al. Loss of heterozygosity of essential genes represents a widespread class of potential cancer vulnerabilities. Nat. Commun. 11, 2517 (2020).
Hwang, M. S. et al. Targeting loss of heterozygosity for cancer-specific immunotherapy. Proc. Natl Acad. Sci. USA 118, e2022410118 (2021).
Laurent, A. P., Kotecha, R. S. & Malinge, S. Gain of chromosome 21 in hematological malignancies: lessons from studying leukemia in children with Down syndrome. Leukemia 34, 1984–1999 (2020).
Hasle, H., Clemmensen, I. H. & Mikkelsen, M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet 355, 165–169 (2000).
Wechsler, J. et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat. Genet. 32, 148–152 (2002).
Hitzler, J. K., Cheung, J., Li, Y., Scherer, S. W. & Zipursky, A. GATA1 mutations in transient leukemia and acute megakaryoblastic leukemia of Down syndrome. Blood 101, 4301–4304 (2003).
Mundschau, G. et al. Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood 101, 4298–4300 (2003).
Yoshida, K. et al. The landscape of somatic mutations in Down syndrome-related myeloid disorders. Nat. Genet. 45, 1293–1299 (2013).
Tunstall-Pedoe, O. et al. Abnormalities in the myeloid progenitor compartment in Down syndrome fetal liver precede acquisition of GATA1 mutations. Blood 112, 4507–4511 (2008).
Roy, A. et al. Perturbation of fetal liver hematopoietic stem and progenitor cell development by trisomy 21. Proc. Natl Acad. Sci. USA 109, 17579–17584 (2012).
Banno, K. et al. Systematic cellular disease models reveal synergistic interaction of trisomy 21 and GATA1 mutations in hematopoietic abnormalities. Cell Rep. 15, 1228–1241 (2016).
Liu, G. et al. Gene essentiality is a quantitative property linked to cellular evolvability. Cell 163, 1388–1399 (2015).
van Leeuwen, J. et al. Systematic analysis of bypass suppression of essential genes. Mol. Syst. Biol. 16, e9828 (2020).
Haigis, K. M. & Sweet-Cordero, A. New insights into oncogenic stress. Nat. Genet. 43, 177–178 (2011).
Raulet, D. H. & Guerra, N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat. Rev. Immunol. 9, 568–580 (2009).
Su, X. A. et al. RAD21 is a driver of chromosome 8 gain in Ewing sarcoma to mitigate replication stress. Genes Dev. 35, 556–572 (2021).
Woo, R. A. & Poon, R. Y. Activated oncogenes promote and cooperate with chromosomal instability for neoplastic transformation. Genes Dev. 18, 1317–1330 (2004).
Denko, N. C., Giaccia, A. J., Stringer, J. R. & Stambrook, P. J. The human Ha-ras oncogene induces genomic instability in murine fibroblasts within one cell cycle. Proc. Natl Acad. Sci. USA 91, 5124–5128 (1994).
Sotillo, R., Schvartzman, J. M., Socci, N. D. & Benezra, R. Mad2-induced chromosome instability leads to lung tumour relapse after oncogene withdrawal. Nature 464, 436–440 (2010).
Salgueiro, L. et al. Acquisition of chromosome instability is a mechanism to evade oncogene addiction. EMBO Mol. Med. 12, e10941 (2020).
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012).
Burrell, R. A., McGranahan, N., Bartek, J. & Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 501, 338–345 (2013).
Teixeira, V. H. et al. Deciphering the genomic, epigenomic, and transcriptomic landscapes of pre-invasive lung cancer lesions. Nat. Med. 25, 517–525 (2019).
Sikkema, M. et al. Aneuploidy and overexpression of Ki67 and p53 as markers for neoplastic progression in Barrett’s esophagus: a case-control study. Am. J. Gastroenterol. 104, 2673–2680 (2009).
Hadjinicolaou, A. V. et al. Aneuploidy in targeted endoscopic biopsies outperforms other tissue biomarkers in the prediction of histologic progression of Barrett’s oesophagus: a multi-centre prospective cohort study. EBioMedicine 56, 102765 (2020).
Melsheimer, P., Vinokurova, S., Wentzensen, N., Bastert, G. & von Knebel Doeberitz, M. DNA aneuploidy and integration of human papillomavirus type 16 e6/e7 oncogenes in intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix uteri. Clin. Cancer Res. 10, 3059–3063 (2004).
Rubin, C. E. et al. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology 103, 1611–1620 (1992).
Hirano, T., Franzén, B., Kato, H., Ebihara, Y. & Auer, G. Genesis of squamous cell lung carcinoma. Sequential changes of proliferation, DNA ploidy, and p53 expression. Am. J. Pathol. 144, 296–302 (1994).
Smith, A. L. et al. Extensive areas of aneuploidy are present in the respiratory epithelium of lung cancer patients. Br. J. Cancer 73, 203–209 (1996).
Molina, O., Abad, M. A., Solé, F. & Menéndez, P. Aneuploidy in cancer: lessons from acute lymphoblastic leukemia. Trends Cancer 7, 37–47 (2021).
Gordon, D. J., Resio, B. & Pellman, D. Causes and consequences of aneuploidy in cancer. Nat. Rev. Genet. 13, 189–203 (2012).
Brennan, C. W. et al. The somatic genomic landscape of glioblastoma. Cell 155, 462–477 (2013).
Bredel, M. et al. A network model of a cooperative genetic landscape in brain tumors. JAMA 302, 261–275 (2009).
Körber, V. et al. Evolutionary trajectories of IDH(WT) glioblastomas reveal a common path of early tumorigenesis instigated years ahead of initial diagnosis. Cancer Cell 35, 692–704.e12 (2019).
Sack, L. M. et al. Profound tissue specificity in proliferation control underlies cancer drivers and aneuploidy patterns. Cell 173, 499–514.e23 (2018).
Davoli, T. et al. Cumulative haploinsufficiency and triplosensitivity drive aneuploidy patterns and shape the cancer genome. Cell 155, 948–962 (2013).
Bielski, C. M. et al. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat. Genet. 50, 1189–1195 (2018).
Watkins, T. B. K. et al. Pervasive chromosomal instability and karyotype order in tumour evolution. Nature 587, 126–132 (2020).
Sottoriva, A. et al. A Big Bang model of human colorectal tumor growth. Nat. Genet. 47, 209–216 (2015).
Gao, R. et al. Punctuated copy number evolution and clonal stasis in triple-negative breast cancer. Nat. Genet. 48, 1119–1130 (2016).
Minussi, D. C. et al. Breast tumours maintain a reservoir of subclonal diversity during expansion. Nature 592, 302–308 (2021).
Navin, N. et al. Tumour evolution inferred by single-cell sequencing. Nature 472, 90–94 (2011).
Turajlic, S. et al. Tracking cancer evolution reveals constrained routes to metastases: TRACERx Renal. Cell 173, 581–594.e12 (2018).
Lukow, D. A. et al. Chromosomal instability accelerates the evolution of resistance to anti-cancer therapies. Dev. Cell 56, 2427–2439.e4 (2021).
Ippolito, M. R. et al. Gene copy-number changes and chromosomal instability induced by aneuploidy confer resistance to chemotherapy. Dev. Cell 56, 2440–2454.e6 (2021).
Kwong, L. N. et al. Modeling genomic instability and selection pressure in a mouse model of melanoma. Cell Rep. 19, 1304–1312 (2017).
Shoshani, O. et al. Transient genomic instability drives tumorigenesis through accelerated clonal evolution. Genes Dev. 35, 1093–1108 (2021).
Bollen, Y. et al. Reconstructing single-cell karyotype alterations in colorectal cancer identifies punctuated and gradual diversification patterns. Nat. Genet. 53, 1187–1195 (2021).
Niccoli, T. & Partridge, L. Ageing as a risk factor for disease. Curr. Biol. 22, R741–R752 (2012).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Wiktor, A. et al. Clinical significance of Y chromosome loss in hematologic disease. Genes. Chromosomes Cancer 27, 11–16 (2000).
Machiela, M. J. et al. Female chromosome X mosaicism is age-related and preferentially affects the inactivated X chromosome. Nat. Commun. 7, 11843 (2016).
Guttenbach, M., Koschorz, B., Bernthaler, U., Grimm, T. & Schmid, M. Sex chromosome loss and aging: in situ hybridization studies on human interphase nuclei. Am. J. Hum. Genet. 57, 1143–1150 (1995).
Ly, D. H., Lockhart, D. J., Lerner, R. A. & Schultz, P. G. Mitotic misregulation and human aging. Science 287, 2486–2492 (2000).
Macedo, J. C. et al. FoxM1 repression during human aging leads to mitotic decline and aneuploidy-driven full senescence. Nat. Commun. 9, 2834 (2018).
Barroso-Vilares, M. et al. Small-molecule inhibition of aging-associated chromosomal instability delays cellular senescence. EMBO Rep. 21, e49248 (2020).
Barger, C. J., Branick, C., Chee, L. & Karpf, A. R. Pan-cancer analyses reveal genomic features of FOXM1 overexpression in cancer. Cancers 11, 251 (2019).
Hu, Z. et al. Nucleosome loss leads to global transcriptional up-regulation and genomic instability during yeast aging. Genes Dev. 28, 396–408 (2014).
Franasiak, J. M. et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil. Steril. 101, 656–663.e651 (2014).
Esbensen, A. J. Health conditions associated with aging and end of life of adults with Down syndrome. Int. Rev. Res. Ment. Retard. 39, 107–126 (2010).
Baker, D. J. et al. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 36, 744–749 (2004).
Baker, D. J. et al. Increased expression of BubR1 protects against aneuploidy and cancer and extends healthy lifespan. Nat. Cell Biol. 15, 96–102 (2013).
Naylor, R. M. & van Deursen, J. M. Aneuploidy in cancer and aging. Annu. Rev. Genet. 50, 45–66 (2016).
Andriani, G. A. et al. Whole chromosome instability induces senescence and promotes SASP. Sci. Rep. 6, 35218 (2016).
He, Q. et al. Chromosomal instability-induced senescence potentiates cell non-autonomous tumourigenic effects. Oncogenesis 7, 62 (2018).
Foijer, F. et al. Spindle checkpoint deficiency is tolerated by murine epidermal cells but not hair follicle stem cells. Proc. Natl Acad. Sci. USA 110, 2928–2933 (2013).
Mirkovic, M., Guilgur, L. G., Tavares, A., Passagem-Santos, D. & Oliveira, R. A. Induced aneuploidy in neural stem cells triggers a delayed stress response and impairs adult life span in flies. PLoS Biol. 17, e3000016 (2019).
Gogendeau, D. et al. Aneuploidy causes premature differentiation of neural and intestinal stem cells. Nat. Commun. 6, 8894 (2015).
Peter, J. et al. Genome evolution across 1,011 Saccharomyces cerevisiae isolates. Nature 556, 339–344 (2018).
Mannaert, A., Downing, T., Imamura, H. & Dujardin, J. C. Adaptive mechanisms in pathogens: universal aneuploidy in Leishmania. Trends Parasitol. 28, 370–376 (2012).
Duan, S. F. et al. The origin and adaptive evolution of domesticated populations of yeast from Far East Asia. Nat. Commun. 9, 2690 (2018).
Bianconi, E. et al. An estimation of the number of cells in the human body. Ann. Hum. Biol. 40, 463–471 (2013).
Thompson, S. L. & Compton, D. A. Proliferation of aneuploid human cells is limited by a p53-dependent mechanism. J. Cell Biol. 188, 369–381 (2010).
Soto, M. et al. p53 prohibits propagation of chromosome segregation errors that produce structural aneuploidies. Cell Rep. 19, 2423–2431 (2017).
Santaguida, S. et al. Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the immune system. Dev. Cell 41, 638–651.e5 (2017).
Narkar, A. et al. On the role of p53 in the cellular response to aneuploidy. Cell Rep. 34, 108892 (2021).
Harding, S. M. et al. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 548, 466–470 (2017).
Mackenzie, K. J. et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 548, 461–465 (2017).
Flynn, P. J., Koch, P. D. & Mitchison, T. J. Chromatin bridges, not micronuclei, activate cGAS after drug-induced mitotic errors in human cells. Proc. Natl Acad. Sci. USA 118, e2103585118 (2021).
Wang, R. W., Viganò, S., Ben-David, U., Amon, A. & Santaguida, S. Aneuploid senescent cells activate NF-κB to promote their immune clearance by NK cells. EMBO Rep. 22, e52032 (2021).
Ji, Z., Chuen, J., Kiparaki, M. & Baker, N. Cell competition removes segmental aneuploid cells from Drosophila imaginal disc-derived tissues based on ribosomal protein gene dose. eLife 10, e61172 (2021).
Kaya, A. et al. Adaptive aneuploidy protects against thiol peroxidase deficiency by increasing respiration via key mitochondrial proteins. Proc. Natl Acad. Sci. USA 112, 10685–10690 (2015).
Ryu, H. Y., Wilson, N. R., Mehta, S., Hwang, S. S. & Hochstrasser, M. Loss of the SUMO protease Ulp2 triggers a specific multichromosome aneuploidy. Genes Dev. 30, 1881–1894 (2016).
Ryu, H. Y. et al. Distinct adaptive mechanisms drive recovery from aneuploidy caused by loss of the Ulp2 SUMO protease. Nat. Commun. 9, 5417 (2018).
Janbon, G., Sherman, F. & Rustchenko, E. Monosomy of a specific chromosome determines L-sorbose utilization: a novel regulatory mechanism in Candida albicans. Proc. Natl Acad. Sci. USA 95, 5150–5155 (1998).
Kabir, M. A., Ahmad, A., Greenberg, J. R., Wang, Y. K. & Rustchenko, E. Loss and gain of chromosome 5 controls growth of Candida albicans on sorbose due to dispersed redundant negative regulators. Proc. Natl Acad. Sci. USA 102, 12147–12152 (2005).
Selmecki, A. M., Dulmage, K., Cowen, L. E., Anderson, J. B. & Berman, J. Acquisition of aneuploidy provides increased fitness during the evolution of antifungal drug resistance. PLoS Genet. 5, e1000705 (2009).
Sionov, E., Lee, H., Chang, Y. C. & Kwon-Chung, K. J. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog. 6, e1000848 (2010).
Stone, N. R. et al. Dynamic ploidy changes drive fluconazole resistance in human cryptococcal meningitis. J. Clin. Invest. 129, 999–1014 (2019).
Prieto Barja, P. et al. Haplotype selection as an adaptive mechanism in the protozoan pathogen Leishmania donovani. Nat. Ecol. Evol. 1, 1961–1969 (2017).
Downing, T. et al. Whole genome sequencing of multiple Leishmania donovani clinical isolates provides insights into population structure and mechanisms of drug resistance. Genome Res. 21, 2143–2156 (2011).
Bussotti, G. et al. Leishmania genome dynamics during environmental adaptation reveal strain-specific differences in gene copy number variation, karyotype instability, and telomeric amplification. mBio 9, e01399-18 (2018).
Liu, X. et al. Trisomy eight in ES cells is a common potential problem in gene targeting and interferes with germ line transmission. Dev. Dyn. 209, 85–91 (1997).
Ben-David, U. et al. Aneuploidy induces profound changes in gene expression, proliferation and tumorigenicity of human pluripotent stem cells. Nat. Commun. 5, 4825 (2014).
Rutledge, S. D. et al. Selective advantage of trisomic human cells cultured in non-standard conditions. Sci. Rep. 6, 22828 (2016).
Hansemann, D. Ueber asymmetrische Zelltheilung in Epithelkrebsen und deren biologische Bedeutung. Archiv f. pathol. Anat. 119, 299–326 (1890).
Boveri, T. Concerning the origin of malignant tumours by Theodor Boveri. translated and annotated by Henry Harris. J. Cell Sci. 121, 1–84 (2008).
Bridges, C. B. Non-disjunction as proof of the chromosome theory of heredity. Genetics 1, 1–52 (1916).
Blakeslee, A. F. Variations in Datura due to changes in chromosome number. Am. Nat. 56, 16–31 (1922).
Tjio, J. H. & Levan, A. The chromosome number of man. Hereditas 42, 1–6 (1956).
Rowley, J. D. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature 243, 290–293 (1973).
White, B. J., Tjio, J. H., Van de Water, L. C. & Crandall, C. Trisomy 19 in the laboratory mouse. I. Frequency in different crosses at specific developmental stages and relationship of trisomy to cleft palate. Cytogenet. Cell Genet. 13, 217–231 (1974).
Mitelman, F. Catalogue of chromosome aberrations in cancer. Cytogenet. Cell Genet. 36, 1–515 (1983).
Cuckle, H. S., Wald, N. J. & Lindenbaum, R. H. Maternal serum alpha-fetoprotein measurement: a screening test for Down syndrome. Lancet 1, 926–929 (1984).
Merkatz, I. R., Nitowsky, H. M., Macri, J. N. & Johnson, W. E. An association between low maternal serum alpha-fetoprotein and fetal chromosomal abnormalities. Am. J. Obstet. Gynecol. 148, 886–894 (1984).
Hoyt, M. A., Totis, L. & Roberts, B. T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66, 507–517 (1991).
Li, R. & Murray, A. W. Feedback control of mitosis in budding yeast. Cell 66, 519–531 (1991).
Schvartzman, J.-M., Sotillo, R. & Benezra, R. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat. Rev. Cancer 10, 102–115 (2010).
Acknowledgements
The authors thank A. Holland (Johns Hopkins University School of Medicine) and M. Gordon (Johns Hopkins University School of Medicine) for valuable comments on the manuscript.
Author information
Authors and Affiliations
Contributions
Both authors contributed significantly to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Molecular Cell Biology thanks Marie-Hélène Verlhac, Todd Stukenberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Trisomy 21
-
(T21). The genetic abnormality characterized by an extra copy of chromosome 21 that causes Down syndrome, the most common chromosomal disorder, affecting roughly 1 in 700 human babies born.
- Cellular senescence
-
Irreversible exist from the cell cycle usually in response to diverse stress.
- Euploid
-
The state of having complete sets of chromosomes in eukaryotic cells.
- Merotelic attachments
-
Erroneous kinetochore–microtubule attachments where one kinetochore is attached to microtubules from both spindle poles.
- Pericentriolar material
-
A complex set of proteins surrounding the centrioles that harbour the microtubule-nucleating activity of centrosomes.
- Cohesin
-
A ring-like structure that is loaded onto chromosomes at the time of DNA replication and maintains cohesion between sister chromotids until chromosome segregation occurs.
- Mosaic loss
-
Chromosomal loss events resulting in mosaicism.
- Replication forks
-
Structures organized by helicase and other DNA replication-related proteins that allow replication of each strand.
- Ultrafine DNA bridges
-
(UFBs). Thin DNA structures that link separating sister chromatids in mitosis.
- R loops
-
Nucleic acid structures consisting of a DNA–RNA hybrid and a displaced single-stranded DNA that normally form during transcription and DNA replication but that are also present in mitotic cells.
- X chromosome inactivation
-
The phenomenon of random inactivation of one of the two X chromosomes during development in females.
- CpG sites
-
Regions of DNA where a cytosine (C) is followed by a guanine (G) and the cytosine is frequently methylated in mammals.
- DNA methyltransferase DNMT3L
-
A member of the DNA methyltransferase family that lacks a catalytic domain and functions as a stimulating cofactor for DNA methylation.
- HMGN1
-
A nucleosomal DNA-binding protein that modulates open chromatin structure and promotes transcription.
- Sir2
-
The yeast nicotinamide adenine dinucleotide (NAD)-dependent protein deacetylase that catalyses the removal of acetyl groups from acetyllysine residues.
- Subtelomeric regions
-
Regions on the chromosome of unique DNA sequence characteristics that are immediately adjacent to the telomeres, specialized structures defining chromosome ends.
- Myosin II
-
A conserved actin-based motor protein that drives cytokinetic ring contraction in diverse eukaryotic cells.
- Environmental stress response
-
A common yeast gene expression programme involving ~900 genes responding to multiple stresses.
- Translesion synthesis
-
A process in which specialized DNA polymerases synthesize a short patch of DNA to bypass blocking DNA lesions.
- Chromothripsis
-
Massive chromosome rearrangements that result in abnormal chromosomes containing multiple segments from different chromosomes.
- Aggresome
-
An intracellular structures made up of aggregated misfolded proteins.
- Unfolded protein response
-
An integrated intracellular signalling pathway responding to the burden of unfolded proteins in the endoplasmic reticulum lumen.
- Somatic evolution
-
Genetic and epigenetic changes in the clonal composition of somatic cells in tissues over time resulting from evolutionary selection.
- Acute megakaryoblastic leukaemia
-
A subtype of acute myeloid leukaemia characterized by abnormal megakaryoblasts.
- Clonogenicity
-
The ability of cells to grow and form colonies.
- Oncogene addiction
-
Dependence of cancers on specific oncogenic mutations to survive and/or proliferate.
- Triple-negative breast cancers
-
Breast cancers that test negative for oestrogen receptors, progesterone receptors and excess HER2 protein.
- Adult stem cells
-
Stem cells in adult tissues that are capable of self-renewal and differentiate into more specialized cell types.
- cGAS–STING pathway
-
A cytosolic DNA sensing mechanism that triggers inflammatory responses.
- Innate immune system
-
Part of the immune system that forms the first line of defence against pathogens (consisting of physical, chemical and cellular mechanisms).
Rights and permissions
About this article
Cite this article
Li, R., Zhu, J. Effects of aneuploidy on cell behaviour and function. Nat Rev Mol Cell Biol 23, 250–265 (2022). https://doi.org/10.1038/s41580-021-00436-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41580-021-00436-9
This article is cited by
-
Full-spectral genome analysis of natural killer/T cell lymphoma highlights impacts of genome instability in driving its progression
Genome Medicine (2024)
-
Aneuploid embryonic stem cells drive teratoma metastasis
Nature Communications (2024)
-
Large-scale genomic rearrangements boost SCRaMbLE in Saccharomyces cerevisiae
Nature Communications (2024)
-
Meiotic and mitotic aneuploidies drive arrest of in vitro fertilized human preimplantation embryos
Genome Medicine (2023)
-
Population monitoring of trisomy 21: problems and approaches
Molecular Cytogenetics (2023)