Abstract
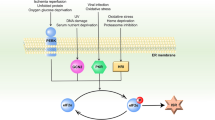
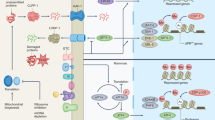
Healthy aging requires the coordination of numerous stress signaling pathways that converge on the protein homeostasis network. The integrated stress response (ISR) is activated by diverse stimuli, leading to phosphorylation of the eukaryotic translation initiation factor eIF2 in its α-subunit. Under replete conditions, eIF2 orchestrates 5′ cap–dependent mRNA translation and is thus responsible for general protein synthesis. eIF2α phosphorylation, the key event of the ISR, reduces global mRNA translation while enhancing the expression of a signature set of stress response genes. Despite the critical role of protein quality control in healthy aging and in numerous longevity pathways, the role of the ISR in longevity remains largely unexplored. ISR activity increases with age, suggesting a potential link with the aging process. Although decreased protein biosynthesis, which occurs during ISR activation, has been linked to lifespan extension, recent data show that lifespan is limited by the ISR as its inhibition extends survival in nematodes and enhances cognitive function in aged mice. Here we survey how aging affects the ISR, the role of the ISR in modulating aging, and pharmacological interventions to tune the ISR. Finally, we will explore the ISR as a plausible target for clinical interventions in aging and age-related disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
The Lancet Diabetes & Endocrinology. Opening the door to treating ageing as a disease. Lancet Diabetes Endocrinol. 6, 587 (2018).
López-Otín, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. The hallmarks of aging. Cell 153, 1194–1217 (2013).
Kourtis, N. & Tavernarakis, N. Cellular stress response pathways and ageing: intricate molecular relationships. EMBO J. 30, 2520–2531 (2011).
Kyriakakis, E., Princz, A. & Tavernarakis, N. Stress responses during ageing: molecular pathways regulating protein homeostasis. Methods Mol. Biol. 1292, 215–234 (2015).
Klaips, C. L., Jayaraj, G. G. & Hartl, F. U. Pathways of cellular proteostasis in aging and disease. J. Cell Biol. 217, 51–63 (2018).
Dever, T. E. et al. Phosphorylation of initiation factor 2α by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell 68, 585–596 (1992).
Brostrom, C. O., Prostko, C. R., Kaufman, R. J. & Brostrom, M. A. Inhibition of translational initiation by activators of the glucose-regulated stress protein and heat shock protein stress response systems. Role of the interferon-inducible double-stranded RNA-activated eukaryotic initiation factor 2α kinase. J. Biol. Chem. 271, 24995–25002 (1996).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Merrick, W. C. & Pavitt, G. D. Protein synthesis initiation in eukaryotic cells. Cold Spring Harb. Perspect. Biol. 10, a033092 (2018).
Lomakin, I. B. & Steitz, T. A. The initiation of mammalian protein synthesis and mRNA scanning mechanism. Nature 500, 307–311 (2013).
Pavitt, G. D., Ramaiah, K. V., Kimball, S. R. & Hinnebusch, A. G. eIF2 independently binds two distinct eIF2B subcomplexes that catalyze and regulate guanine-nucleotide exchange. Genes Dev. 12, 514–526 (1998).
Han, A.-P. et al. Heme-regulated eIF2α kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J. 20, 6909–6918 (2001).
Kumar, K. U., Srivastava, S. P. & Kaufman, R. J. Double-stranded RNA-activated protein kinase (PKR) is negatively regulated by 60S ribosomal subunit protein L18. Mol. Cell. Biol. 19, 1116–1125 (1999).
Shi, Y. et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18, 7499–7509 (1998).
Guo, X. et al. Mitochondrial stress is relayed to the cytosol by an OMA1–DELE1–HRI pathway. Nature 579, 427–432 (2020).
Fessler, E. et al. A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol. Nature 579, 433–437 (2020).
Taniuchi, S., Miyake, M., Tsugawa, K., Oyadomari, M. & Oyadomari, S. Integrated stress response of vertebrates is regulated by four eIF2α kinases. Sci. Rep. 6, 32886 (2016).
Kimball, S. R., Fabian, J. R., Pavitt, G. D., Hinnebusch, A. G. & Jefferson, L. S. Regulation of guanine nucleotide exchange through phosphorylation of eukaryotic initiation factor eIF2α. Role of the α- and δ-subunits of eiF2b. J. Biol. Chem. 273, 12841–12845 (1998).
Adomavicius, T. et al. The structural basis of translational control by eIF2 phosphorylation. Nat. Commun. 10, 2136 (2019).
Gordiyenko, Y., Llácer, J. L. & Ramakrishnan, V. Structural basis for the inhibition of translation through eIF2α phosphorylation. Nat. Commun. 10, 2640 (2019).
Vattem, K. M. & Wek, R. C. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl Acad. Sci. USA 101, 11269–11274 (2004).
Sidrauski, C., McGeachy, A. M., Ingolia, N. T. & Walter, P. The small molecule ISRIB reverses the effects of eIF2α phosphorylation on translation and stress granule assembly. Elife 4, e05033 (2015).
Lee, Y.-Y., Cevallos, R. C. & Jan, E. An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2α phosphorylation. J. Biol. Chem. 284, 6661–6673 (2009).
Jousse, C. et al. Inhibition of CHOP translation by a peptide encoded by an open reading frame localized in the chop 5′UTR. Nucl. Acids Res. 29, 4341–4351 (2001).
Jousse, C. et al. Inhibition of a constitutive translation initiation factor 2α phosphatase, CReP, promotes survival of stressed cells. J. Cell Biol. 163, 767–775 (2003).
Novoa, I., Zeng, H., Harding, H. P. & Ron, D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2α. J. Cell Biol. 153, 1011–1022 (2001).
Rutkowski, D. T. et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 4, e374 (2006).
Han, J. et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 15, 481–490 (2013).
Marciniak, S. J. et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 18, 3066–3077 (2004).
Frakes, A. E. & Dillin, A. The UPRER: sensor and coordinator of organismal homeostasis. Mol. Cell 66, 761–771 (2017).
Cullinan, S. B. et al. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 23, 7198–7209 (2003).
Ben-Sahra, I., Hoxhaj, G., Ricoult, S. J. H., Asara, J. M. & Manning, B. D. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science 351, 728–733 (2016).
Torrence, M. E. et al. The mTORC1-mediated activation of ATF4 promotes protein and glutathione synthesis downstream of growth signals. Elife 10, (2021).
McConkey, D. J. The integrated stress response and proteotoxicity in cancer therapy. Biochem. Biophys. Res. Commun. 482, 450–453 (2017).
Moortgat, S. et al. Two novel EIF2S3 mutations associated with syndromic intellectual disability with severe microcephaly, growth retardation, and epilepsy. Am. J. Med. Genet. A 170, 2927–2933 (2016).
Skopkova, M. et al. EIF2S3 mutations associated with severe X-linked intellectual disability syndrome MEHMO. Hum. Mutat. 38, 409–425 (2017).
Nguyen, H. G. et al. Development of a stress response therapy targeting aggressive prostate cancer. Sci. Transl. Med. 10, eaar2036 (2018).
Tameire, F. et al. ATF4 couples MYC-dependent translational activity to bioenergetic demands during tumour progression. Nat. Cell Biol. 21, 889–899 (2019).
Chou, A. et al. Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Proc. Natl Acad. Sci. USA 114, E6420–E6426 (2017).
Zhu, P. J. et al. Activation of the ISR mediates the behavioral and neurophysiological abnormalities in Down syndrome. Science 366, 843–849 (2019).
Bugallo, R. et al. Fine tuning of the unfolded protein response by ISRIB improves neuronal survival in a model of amyotrophic lateral sclerosis. Cell Death Dis. 11, 397 (2020).
Oliveira, M. M. et al. Correction of eIF2-dependent defects in brain protein synthesis, synaptic plasticity, and memory in mouse models of Alzheimer’s disease. Sci. Signal. 14, eabc5429 (2021).
Colla, E. et al. Accumulation of toxic α-synuclein oligomer within endoplasmic reticulum occurs in α-synucleinopathy in vivo. J. Neurosci. 32, 3301–3305 (2012).
Jiang, H.-Q. et al. Guanabenz delays the onset of disease symptoms, extends lifespan, improves motor performance and attenuates motor neuron loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neuroscience 277, 132–138 (2014).
Özcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Scali, O., Di Perri, C. & Federico, A. The spectrum of mutations for the diagnosis of vanishing white matter disease. Neurol. Sci. 27, 271–277 (2006).
Hanefeld, F. et al. Diffuse white matter disease in three children: an encephalopathy with unique features on magnetic resonance imaging and proton magnetic resonance spectroscopy. Neuropediatrics 24, 244–248 (1993).
Schiffmann, R. et al. Childhood ataxia with diffuse central nervous system hypomyelination. Ann. Neurol. 35, 331–340 (1994).
Mao, D. et al. De novo EIF2AK1 and EIF2AK2 variants are associated with developmental delay, leukoencephalopathy, and neurologic decompensation. Am. J. Hum. Genet. 106, 570–583 (2020).
DeLozier-Blanchet, C. D., Haenggeli, C. A. & Bottani, A. MEHMO, a novel syndrome: assignment of disease locus to Xp21.1-p22.13. Mental retardation, epileptic seizures, hypogonadism and genitalism, microcephaly, obesity. Eur. J. Hum. Genet. 7, 621–622 (1999).
Hunter, J. M. et al. Review of X-linked syndromes with arthrogryposis or early contractures—aid to diagnosis and pathway identification. Am. J. Med. Genet. A 167A, 931–973 (2015).
Gregory, L. C. et al. Impaired EIF2S3 function associated with a novel phenotype of X-linked hypopituitarism with glucose dysregulation. EBioMedicine 42, 470–480 (2019).
Abdulkarim, B. et al. A missense mutation in PPP1R15B causes a syndrome including diabetes, short stature, and microcephaly. Diabetes 64, 3951–3962 (2015).
Kernohan, K. D. et al. Homozygous mutation in the eukaryotic translation initiation factor 2α phosphatase gene, PPP1R15B, is associated with severe microcephaly, short stature and intellectual disability. Hum. Mol. Genet. 24, 6293–6300 (2015).
Wolcott, C. D. & Rallison, M. L. Infancy-onset diabetes mellitus and multiple epiphyseal dysplasia. J. Pediatr. 80, 292–297 (1972).
Julier, C. & Nicolino, M. Wolcott-Rallison syndrome. Orphanet J. Rare Dis. 5, 29–13 (2010).
Eyries, M. et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat. Genet. 46, 65–69 (2014).
Naidoo, N. et al. Aging and sleep deprivation induce the unfolded protein response in the pancreas: implications for metabolism. Aging Cell 13, 131–141 (2014).
Ladiges, W., Morton, J., Blakely, C. & Gale, M. Tissue specific expression of PKR protein kinase in aging B6D2F1 mice. Mech. Ageing Dev. 114, 123–132 (2000).
Ubaida-Mohien, C. et al. Discovery proteomics in aging human skeletal muscle finds change in spliceosome, immunity, proteostasis and mitochondria. Elife 8, 852 (2019).
Krukowski, K. et al. Small molecule cognitive enhancer reverses age-related memory decline in mice. Elife 9, 1671 (2020).
Chalil, S. et al. Aging related ER stress is not responsible for anabolic resistance in mouse skeletal muscle. Biochem. Biophys. Res. Commun. 468, 702–707 (2015).
Segev, Y., Michaelson, D. M. & Rosenblum, K. ApoE ε4 is associated with eIF2α phosphorylation and impaired learning in young mice. Neurobiol. Aging 34, 863–872 (2013).
Brown, M. K. et al. Aging induced endoplasmic reticulum stress alters sleep and sleep homeostasis. Neurobiol. Aging 35, 1431–1441 (2014).
Derisbourg, M. J., Wester, L. E., Baddi, R. & Denzel, M. S. Mutagenesis screen uncovers lifespan extension through integrated stress response inhibition without reduced mRNA translation. Nat. Commun. 12, 1678 (2021).
Salganik, M. et al. The loss of glucose-regulated protein 78 (GRP78) during normal aging or from siRNA knockdown augments human alpha-synuclein (α-syn) toxicity to rat nigral neurons. Neurobiol. Aging 36, 2213–2223 (2015).
Li, W., Li, X. & Miller, R. A. ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell 13, 1012–1018 (2014).
Li, W. & Miller, R. A. Elevated ATF4 function in fibroblasts and liver of slow-aging mutant mice. J. Gerontol. A Biol. Sci. Med. Sci. 70, 263–272 (2015).
Makrides, S. C. Protein synthesis and degradation during aging and senescence. Biol. Rev. 58, 343–422 (1983).
Johnson, T. E. & McCaffrey, G. Programmed aging or error catastrophe? An examination by two-dimensional polyacrylamide gel electrophoresis. Mech. Ageing Dev. 30, 285–297 (1985).
Rattan, S. I. & Clark, B. F. Intracellular protein synthesis, modifications and aging. Biochem. Soc. Trans. 24, 1043–1049 (1996).
Tavernarakis, N. Ageing and the regulation of protein synthesis: a balancing act? Trends Cell Biol. 18, 228–235 (2008).
Iwawaki, T. et al. Transgenic mouse model for imaging of ATF4 translational activation-related cellular stress responses in vivo. Sci. Rep. 7, 46230–46239 (2017).
Helseth, A. R. et al. Cholinergic neurons constitutively engage the ISR for dopamine modulation and skill learning in mice. Science https://doi.org/10.1126/science.abe1931 (2021).
Wu, C. C.-C., Peterson, A., Zinshteyn, B., Regot, S. & Green, R. Ribosome collisions trigger general stress responses to regulate cell fate. Cell 182, 404–416 (2020).
Peidis, P., Papadakis, A. I., Muaddi, H., Richard, S. & Koromilas, A. E. Doxorubicin bypasses the cytoprotective effects of eIF2α phosphorylation and promotes PKR-mediated cell death. Cell Death Differ. 18, 145–154 (2011).
Hao, S. et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 307, 1776–1778 (2005).
Seo, J. et al. Atf4 regulates obesity, glucose homeostasis, and energy expenditure. Diabetes 58, 2565–2573 (2009).
Ye, J. et al. The GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 29, 2082–2096 (2010).
Rozpedek, W. et al. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 16, 533–544 (2016).
Hu, Z. et al. Ssd1 and Gcn2 suppress global translation efficiency in replicatively aged yeast while their activation extends lifespan. Elife 7, 4443 (2018).
Postnikoff, S. D. L., Johnson, J. E. & Tyler, J. K. The integrated stress response in budding yeast lifespan extension. Microb. Cell 4, 368–375 (2017).
Richardson, C. E., Kinkel, S. & Kim, D. H. Physiological IRE-1-XBP-1 and PEK-1 signaling in Caenorhabditis elegans larval development and immunity. PLoS Genet. 7, e1002391 (2011).
Horsman, J. W. & Miller, D. L. Mitochondrial sulfide quinone oxidoreductase prevents activation of the unfolded protein response in hydrogen sulfide. J. Biol. Chem. 291, 5320–5325 (2016).
Tohyama, D., Yamaguchi, A. & Yamashita, T. Inhibition of a eukaryotic initiation factor (eIF2Bδ/F11A3.2) during adulthood extends lifespan in Caenorhabditis elegans. FASEB J. 22, 4327–4337 (2008).
Horn, M. et al. Hexosamine pathway activation improves protein homeostasis through the integrated stress response. iScience 23, 100887 (2020).
Rousakis, A. et al. The general control nonderepressible-2 kinase mediates stress response and longevity induced by target of rapamycin inactivation in Caenorhabditis elegans. Aging Cell 12, 742–751 (2013).
Shen, X. et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107, 893–903 (2001).
Cassada, R. C. & Russell, R. L. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev. Biol. 46, 326–342 (1975).
Kulalert, W. & Kim, D. H. The unfolded protein response in a pair of sensory neurons promotes entry of C. elegans into dauer diapause. Curr. Biol. 23, 2540–2545 (2013).
Kulalert, W., Sadeeshkumar, H., Zhang, Y. K., Schroeder, F. C. & Kim, D. H. Molecular determinants of the regulation of development and metabolism by neuronal eIF2α phosphorylation in Caenorhabditis elegans. Genetics 206, 251–263 (2017).
Baker, B. M., Nargund, A. M., Sun, T. & Haynes, C. M. Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2. PLoS Genet. 8, e1002760 (2012).
Lee, E. C.-H. & Strange, K. GCN-2 dependent inhibition of protein synthesis activates osmosensitive gene transcription via WNK and Ste20 kinase signaling. Am. J. Physiol. Cell Physiol. 303, C1269–C1277 (2012).
Kim, H. & Strange, K. Changes in translation rate modulate stress-induced damage of diverse proteins. Am. J. Physiol. Cell Physiol. 305, C1257–C1264 (2013).
Garcia-Barrio, M., Dong, J., Ufano, S. & Hinnebusch, A. G. Association of GCN1-GCN20 regulatory complex with the N-terminus of eIF2α kinase GCN2 is required for GCN2 activation. EMBO J. 19, 1887–1899 (2000).
Pereira, C. M. et al. IMPACT, a protein preferentially expressed in the mouse brain, binds GCN1 and inhibits GCN2 activation. J. Biol. Chem. 280, 28316–28323 (2005).
Ferraz, R. C. et al. IMPACT is a GCN2 inhibitor that limits lifespan in Caenorhabditis elegans. BMC Biol. 14, 87 (2016).
Santoyo, J., Alcalde, J., Méndez, R., Pulido, D. & de Haro, C. Cloning and characterization of a cDNA encoding a protein synthesis initiation factor-2α (eIF-2α) kinase from Drosophila melanogaster homology to yeast GCN2 protein kinase. J. Biol. Chem. 272, 12544–12550 (1997).
Olsen, D. S., Jordan, B., Chen, D., Wek, R. C. & Cavener, D. R. Isolation of the gene encoding the Drosophila melanogaster homolog of the Saccharomyces cerevisiae GCN2 eIF-2α kinase. Genetics 149, 1495–1509 (1998).
Pomar, N. et al. Functional characterization of Drosophila melanogaster PERK eukaryotic initiation factor 2α (eIF2α) kinase. Eur. J. Biochem. 270, 293–306 (2003).
Malzer, E. et al. Coordinate regulation of eIF2α phosphorylation by PPP1R15 and GCN2 is required during Drosophila development. J. Cell Sci. 126, 1406–1415 (2013).
Malzer, E. et al. Impaired tissue growth is mediated by checkpoint kinase 1 (CHK1) in the integrated stress response. J. Cell Sci. 123, 2892–2900 (2010).
Wang, L., Ryoo, H. D., Qi, Y. & Jasper, H. PERK limits Drosophila lifespan by promoting intestinal stem cell proliferation in response to ER stress. PLoS Genet. 11, e1005220 (2015).
Kang, M.-J. et al. 4E-BP is a target of the GCN2-ATF4 pathway during Drosophila development and aging. J. Cell Biol. 216, 115–129 (2017).
Scheuner, D. et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165–1176 (2001).
Zhang, P. et al. The PERK eukaryotic initiation factor 2 α kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 22, 3864–3874 (2002).
Harding, H. P. et al. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 α (eIF2α) dephosphorylation in mammalian development. Proc. Natl Acad. Sci. USA 106, 1832–1837 (2009).
Krzyzosiak, A. et al. Target-based discovery of an inhibitor of the regulatory phosphatase PPP1R15B. Cell 174, 1216–1228 (2018).
Das, I. et al. Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 348, 239–242 (2015).
Wang, L., Popko, B., Tixier, E. & Roos, R. P. Guanabenz, which enhances the unfolded protein response, ameliorates mutant SOD1-induced amyotrophic lateral sclerosis. Neurobiol. Dis. 71, 317–324 (2014).
Way, S. W. et al. Pharmaceutical integrated stress response enhancement protects oligodendrocytes and provides a potential multiple sclerosis therapeutic. Nat. Commun. 6, 6532 (2015).
Chen, T. et al. Explorations of substituted urea functionality for the discovery of new activators of the heme-regulated inhibitor kinase. J. Med. Chem. 56, 9457–9470 (2013).
Boyce, M. et al. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science 307, 935–939 (2005).
Costa-Mattioli, M. et al. eIF2α phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129, 195–206 (2007).
Tsaytler, P., Harding, H. P., Ron, D. & Bertolotti, A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science 332, 91–94 (2011).
Carrara, M., Sigurdardottir, A. & Bertolotti, A. Decoding the selectivity of eIF2α holophosphatases and PPP1R15A inhibitors. Nat. Struct. Mol. Biol. 24, 708–716 (2017).
Dedigama-Arachchige, P. M., Acharige, N. P. N. & Pflum, M. K. H. Identification of PP1-Gadd34 substrates involved in the unfolded protein response using K-BIPS, a method for phosphatase substrate identification. Mol. Omics 14, 121–133 (2018).
Crespillo-Casado, A., Chambers, J. E., Fischer, P. M., Marciniak, S. J. & Ron, D. PPP1R15A-mediated dephosphorylation of eIF2α is unaffected by Sephin1 or Guanabenz. Elife 6, 209 (2017).
Crespillo-Casado, A. et al. A Sephin1-insensitive tripartite holophosphatase dephosphorylates translation initiation factor 2α. J. Biol. Chem. 293, 7766–7776 (2018).
Dash, P. K. et al. Inhibition of eukaryotic initiation factor 2 α phosphatase reduces tissue damage and improves learning and memory after experimental traumatic brain injury. J. Neurotrauma 32, 1608–1620 (2015).
Dooves, S. et al. Bergmann glia translocation: a new disease marker for vanishing white matter identifies therapeutic effects of Guanabenz treatment. Neuropathol. Appl. Neurobiol. 44, 391–403 (2018).
Chen, Y. et al. Sephin1, which prolongs the integrated stress response, is a promising therapeutic for multiple sclerosis. Brain 142, 344–361 (2019).
Bella, E. D. et al. The unfolded protein response in amyotrophic later sclerosis: results of a phase 2 trial. Brain https://doi.org/10.1093/brain/awab167 (2021).
Axten, J. M. et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK). J. Med. Chem. 55, 7193–7207 (2012).
Rozpędek, W. et al. Inhibition of PERK-dependent pro-adaptive signaling pathway as a promising approach for cancer treatment. Pol. Przegl. Chir. 89, 7–10 (2017).
Halliday, M. et al. Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis. 6, e1672 (2015).
Moreno, J. A. et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci. Transl. Med. 5, 206ra138 (2013).
Sidrauski, C. et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife 2, e00498 (2013).
Sekine, Y. et al. Mutations in a translation initiation factor identify the target of a memory-enhancing compound. Science 348, 1027–1030 (2015).
Tsai, J. C. Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule. Science 359, eaaq0939 (2018).
Schoof, M. et al. eIF2B conformation and assembly state regulate the integrated stress response. Elife 10, (2021).
Zyryanova, A. F. et al. ISRIB blunts the integrated stress response by allosterically antagonising the inhibitory effect of phosphorylated eIF2 on eIF2B. Mol. Cell 81, 88–103 (2021).
Wong, Y. L. et al. eIF2B activator prevents neurological defects caused by a chronic integrated stress response. Elife 8, 1867 (2019).
Halliday, M. et al. Repurposed drugs targeting eIF2α-P-mediated translational repression prevent neurodegeneration in mice. Brain 140, 1768–1783 (2017).
Radford, H., Moreno, J. A., Verity, N., Halliday, M. & Mallucci, G. R. PERK inhibition prevents tau-mediated neurodegeneration in a mouse model of frontotemporal dementia. Acta Neuropathol. 130, 633–642 (2015).
Briggs, D. I. et al. Role of endoplasmic reticulum stress in learning and memory impairment and Alzheimer’s disease-like neuropathology in the PS19 and APPSwe mouse models of tauopathy and amyloidosis. eNeuro 4, ENEURO.0025-17.2017 (2017).
Johnson, E. C. B. & Kang, J. A small molecule targeting protein translation does not rescue spatial learning and memory deficits in the hAPP-J20 mouse model of Alzheimer’s disease. PeerJ 4, e2565 (2016).
Luh, L. M. & Bertolotti, A. Potential benefit of manipulating protein quality control systems in neurodegenerative diseases. Curr. Opin. Neurobiol. 61, 125–132 (2020).
Anand, A. A. & Walter, P. Structural insights into ISRIB, a memory-enhancing inhibitor of the integrated stress response. FEBS J. 287, 239–245 (2020).
Acknowledgements
We thank all Denzel laboratory members for helpful discussions about this manuscript. We further thank P. Walter, M. Costa-Mattioli and A. Bertolotti for valuable comments on the manuscript. Figure 1 was created with BioRender.com. This work was supported by the European Commission (ERC-2014-StG-640254-MetAGEn) and by the Max Planck Society.
Author information
Authors and Affiliations
Contributions
M.J.D., M.D.H. and M.S.D. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.S.D. is cofounder of Acus Laboratories GmbH and scientific advisor to JLP Health GmbH. All other authors declare no competing interests.
Additional information
Peer review information Nature Aging thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Derisbourg, M.J., Hartman, M.D. & Denzel, M.S. Modulating the integrated stress response to slow aging and ameliorate age-related pathology. Nat Aging 1, 760–768 (2021). https://doi.org/10.1038/s43587-021-00112-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43587-021-00112-9
This article is cited by
-
A comprehensive model for the biochemistry of ageing, senescence and longevity
Biogerontology (2024)
-
Translation dysregulation in neurodegenerative diseases: a focus on ALS
Molecular Neurodegeneration (2023)
-
Functional-metabolic coupling in distinct renal cell types coordinates organ-wide physiology and delays premature ageing
Nature Communications (2023)
-
The transcription regulator ATF4 is a mediator of skeletal muscle aging
GeroScience (2023)
-
Proteomic basis of mortality resilience mediated by FOXO3 longevity genotype
GeroScience (2023)