Abstract
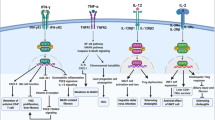
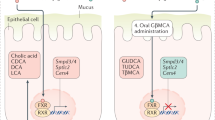
IL-6 family cytokines are defined by the common use of the signal-transducing receptor chain glycoprotein 130 (gp130). Increasing evidence indicates that these cytokines are essential in the regulation of metabolic homeostasis as well as in the pathophysiology of multiple gastrointestinal and liver disorders, thus making them attractive therapeutic targets. Over the past few years, therapies modulating gp130 signalling have grown exponentially in several clinical settings including obesity, cancer and inflammatory bowel disease. A newly engineered gp130 cytokine, IC7Fc, has shown promising preclinical results for the treatment of type 2 diabetes, obesity and liver steatosis. Moreover, drugs that modulate gp130 signalling have shown promise in refractory inflammatory bowel disease in clinical trials. A deeper understanding of the main roles of the IL-6 family of cytokines during homeostatic and pathological conditions, their signalling pathways, sources of production and target cells will be crucial to the development of improved treatments. Here, we review the current state of the role of these cytokines in hepatology and gastroenterology and discuss the progress achieved in translating therapeutics targeting gp130 signalling into clinical practice.
Key points
-
Evidence from animal and human studies supports the role of IL-6 family cytokines in regulating metabolic, hepatic and gastroenterology homeostasis.
-
Activation of gp130 signalling can be detrimental in some contexts and contribute to metabolic, liver and gastrointestinal disorders such as obesity, chronic liver damage, inflammatory bowel disease (IBD) and cancer.
-
Further insights into the involvement of IL-6 family cytokines in homeostasis and the pathophysiology of different disorders are required to develop treatments with adequate risk–benefit profiles.
-
Some therapies targeting gp130 signalling (for example, spg130Fc and JAK inhibitors) are promising novel disease-modifying biological treatments for refractory IBD.
-
Preclinical studies have shown that engineered IL-6 family cytokines such as IC7Fc are promising for the treatment of type 2 diabetes, obesity and liver steatosis.
-
New approaches such as gp130–cytokine fusion proteins, soluble antagonist receptors and more selective or tissue-specific inhibitors of gp130 signalling might improve the effectiveness and safety profile of this class of therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Rose-John, S., Scheller, J. & Schaper, F. “Family reunion”–A structured view on the composition of the receptor complexes of interleukin-6-type and interleukin-12-type cytokines. Cytokine Growth Factor. Rev. 26, 471–474 (2015).
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16, 448–457 (2015).
Garbers, C., Heink, S., Korn, T. & Rose-John, S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat. Rev. Drug Discov. 17, 395–412 (2018).
Findeisen, M. et al. Treatment of type 2 diabetes with the designer cytokine IC7Fc. Nature 574, 63–68 (2019).
Mitsuyama, K. et al. Therapeutic strategies for targeting the IL-6/STAT3 cytokine signaling pathway in inflammatory bowel disease. Anticancer Res. 27, 3749–3756 (2007).
Rose-John, S. Cytokines come of age. Biochim. Biophys. Acta 1592, 213–214 (2002).
Wang, X., Lupardus, P., Laporte, S. L. & Garcia, K. C. Structural biology of shared cytokine receptors. Annu. Rev. Immunol. 27, 29–60 (2009).
Wilmes, S. et al. Mechanism of homodimeric cytokine receptor activation and dysregulation by oncogenic mutations. Science 367, 643–652 (2020).
Garbers, C. et al. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor. Rev. 23, 85–97 (2012).
Grötzinger, J., Kurapkat, G., Wollmer, A., Kalai, M. & Rose-John, S. The family of the IL-6-type cytokines: specificity and promiscuity of the receptor complexes. Proteins 27, 96–109 (1997).
Wilkinson, A. N. et al. Granulocytes are unresponsive to IL-6 due to an absence of gp130. J. Immunol. 200, 3547–3555 (2018).
Jones, S. A. & Jenkins, B. J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 18, 773–789 (2018).
Lokau, J. & Garbers, C. Biological functions and therapeutic opportunities of soluble cytokine receptors. Cytokine Growth Factor Rev. 55, 94–108 (2020).
Riethmueller, S. et al. Proteolytic origin of the soluble human IL-6R in vivo and a decisive role of N-glycosylation. PLoS Biol. 15, e2000080 (2017).
Müllberg, J. et al. The soluble interleukin-6 receptor is generated by shedding. Eur. J. Immunol. 23, 473–480 (1993).
Heink, S. et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic T(H)17 cells. Nat. Immunol. 18, 74–85 (2017).
Garbers, C. & Scheller, J. Interleukin-6 and interleukin-11: same same but different. Biol. Chem. 394, 1145–1161 (2013).
Putoczki, T. & Ernst, M. More than a sidekick: the IL-6 family cytokine IL-11 links inflammation to cancer. J. Leukoc. Biol. 88, 1109–1117 (2010).
Lokau, J. et al. Proteolytic cleavage governs interleukin-11 trans-signaling. Cell Rep. 14, 1761–1773 (2016).
Sun, B. B. et al. Genomic atlas of the human plasma proteome. Nature 558, 73–79 (2018).
Lamertz, L. et al. Soluble gp130 prevents interleukin-6 and interleukin-11 cluster signaling but not intracellular autocrine responses. Sci. Signal 11, eaar7388 (2018).
Heinrich, P. C. et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 (2003).
Schaper, F. & Rose-John, S. Interleukin-6: biology, signaling and strategies of blockade. Cytokine Growth Factor. Rev. 26, 475–487 (2015).
Bastard, J. P. et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J. Clin. Endocrinol. Metab. 85, 3338–3342 (2000).
Steensberg, A. et al. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 529, 237–242 (2000).
Wojtaszewski, J. F. & Richter, E. A. Effects of acute exercise and training on insulin action and sensitivity: focus on molecular mechanisms in muscle. Essays Biochem. 42, 31–46 (2006).
Wallenius, V. et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat. Med. 8, 75–79 (2002).
Matthews, V. B. et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53, 2431–2441 (2010).
Sadagurski, M. et al. Human IL6 enhances leptin action in mice. Diabetologia 53, 525–535 (2010).
Sabio, G. et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 322, 1539–1543 (2008).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-βB. Nat. Med. 11, 183–190 (2005).
Ellingsgaard, H. et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 17, 1481–1489 (2011).
Ellingsgaard, H. et al. GLP-1 secretion is regulated by IL-6 signalling: a randomised, placebo-controlled study. Diabetologia 63, 362–373 (2020).
Han, M. S. et al. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl Acad. Sci. USA 117, 2751–2760 (2020).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).
Yamaguchi, K. et al. Blockade of interleukin 6 signalling ameliorates systemic insulin resistance through upregulation of glucose uptake in skeletal muscle and improves hepatic steatosis in high-fat diet fed mice. Liver Int. 35, 550–561 (2015).
Yamaguchi, K. et al. Blockade of interleukin-6 signaling enhances hepatic steatosis but improves liver injury in methionine choline-deficient diet-fed mice. Lab. Invest. 90, 1169–1178 (2010).
Wunderlich, F. T. et al. Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab. 12, 237–249 (2010).
Mauer, J. et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 15, 423–430 (2014).
Timper, K. et al. IL-6 improves energy and glucose homeostasis in obesity via enhanced central IL-6 trans-signaling. Cell Rep. 19, 267–280 (2017).
Masu, Y. et al. Disruption of the CNTF gene results in motor neuron degeneration. Nature 365, 27–32 (1993).
Pascual-Gamarra, J. M. et al. Association between CNTF polymorphisms and adiposity markers in European adolescents. J. Pediatr. 219, 23–30.e1 (2020).
Watt, M. J. et al. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat. Med. 12, 541–548 (2006).
Zvonic, S., Cornelius, P., Stewart, W. C., Mynatt, R. L. & Stephens, J. M. The regulation and activation of ciliary neurotrophic factor signaling proteins in adipocytes. J. Biol. Chem. 278, 2228–2235 (2003).
Perugini, J. et al. Biological effects of ciliary neurotrophic factor on hMADS adipocytes. Front. Endocrinol. 10, 768 (2019).
Sleeman, M. W. et al. Ciliary neurotrophic factor improves diabetic parameters and hepatic steatosis and increases basal metabolic rate in db/db mice. Proc. Natl Acad. Sci. USA 100, 14297–14302 (2003).
Cui, M. X. et al. Alleviative effect of ciliary neurotrophic factor analogue on high fat-induced hepatic steatosis is partially independent of the central regulation. Clin. Exp. Pharmacol. Physiol. 44, 395–402 (2017).
López-Yoldi, M., Moreno-Aliaga, M. J. & Bustos, M. Cardiotrophin-1: a multifaceted cytokine. Cytokine Growth Factor. Rev. 26, 523–532 (2015).
Castaño, D. et al. Cardiotrophin-1 eliminates hepatic steatosis in obese mice by mechanisms involving AMPK activation. J. Hepatol. 60, 1017–1025 (2014).
Carneros, D. et al. Cardiotrophin-1 is an anti-inflammatory cytokine and promotes IL-4-induced M2 macrophage polarization. FASEB J. 33, 7578–7587 (2019).
Lutz, S. Z. et al. Common genetic variation in the human CTF1 locus, encoding cardiotrophin-1, determines insulin sensitivity. PLoS ONE 9, e100391 (2014).
Rosado-Olivieri, E. A., Aigha, I. I., Kenty, J. H. & Melton, D. A. Identification of a LIF-responsive, replication-competent subpopulation of human β cells. Cell Metab. 31, 327–338.e6 (2020).
Stephens, J., Ravussin, E. & White, U. The expression of adipose tissue-derived cardiotrophin-1 in humans with obesity. Biology 8, 24 (2019).
Brandt, N. et al. Leukemia inhibitory factor increases glucose uptake in mouse skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 309, E142–E153 (2015).
Broholm, C. et al. Deficient leukemia inhibitory factor signaling in muscle precursor cells from patients with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 303, E283–E292 (2012).
Mahajan, A. et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 50, 1505–1513 (2018).
Tanaka, M. et al. Reconstitution of the functional mouse oncostatin M (OSM) receptor: molecular cloning of the mouse OSM receptor beta subunit. Blood 93, 804–815 (1999).
Luo, P. et al. Hepatic oncostatin M receptor β regulates obesity-induced steatosis and insulin resistance. Am. J. Pathol. 186, 1278–1292 (2016).
Henkel, J. et al. Oncostatin M produced in Kupffer cells in response to PGE2: possible contributor to hepatic insulin resistance and steatosis. Lab. Invest. 91, 1107–1117 (2011).
Komori, T., Tanaka, M., Senba, E., Miyajima, A. & Morikawa, Y. Deficiency of oncostatin M receptor β (OSMRβ) exacerbates high-fat diet-induced obesity and related metabolic disorders in mice. J. Biol. Chem. 289, 13821–13837 (2014).
Baumann, H. & Gauldie, J. The acute phase response. Immunol. Today 15, 74–80 (1994).
Espat, N. J. et al. Ciliary neurotrophic factor is catabolic and shares with IL-6 the capacity to induce an acute phase response. Am. J. Physiol. 271, R185–R190 (1996).
Baumann, H. & Schendel, P. Interleukin-11 regulates the hepatic expression of the same plasma protein genes as interleukin-6. J. Biol. Chem. 266, 20424–20427 (1991).
Peters, M., Roeb, E., Pennica, D., Meyer zum Büschenfelde, K. H. & Rose-John, S. A new hepatocyte stimulating factor: cardiotrophin-1 (CT-1). FEBS Lett. 372, 177–180 (1995).
Schooltink, H., Stoyan, T., Roeb, E., Heinrich, P. C. & Rose-John, S. Ciliary neurotrophic factor induces acute-phase protein expression in hepatocytes. FEBS Lett. 314, 280–284 (1992).
Senaldi, G. et al. Novel neurotrophin-1/B cell-stimulating factor-3: a cytokine of the IL-6 family. Proc. Natl Acad. Sci.USA 96, 11458–11463 (1999).
Dittrich, F., Thoenen, H. & Sendtner, M. Ciliary neurotrophic factor: pharmacokinetics and acute-phase response in rat. Ann. Neurol. 35, 151–163 (1994).
Geiger, T. et al. Induction of rat acute-phase proteins by interleukin 6 in vivo. Eur. J. Immunol. 18, 717–721 (1988).
Metcalf, D., Nicola, N. A. & Gearing, D. P. Effects of injected leukemia inhibitory factor on hematopoietic and other tissues in mice. Blood 76, 50–56 (1990).
Wallace, P. M. et al. In vivo properties of oncostatin M. Ann. N. Y. Acad. Sci. 762, 42–54 (1995).
Yonemura, Y., Kawakita, M., Masuda, T., Fujimoto, K. & Takatsuki, K. Effect of recombinant human interleukin-11 on rat megakaryopoiesis and thrombopoiesis in vivo: comparative study with interleukin-6. Br. J. Haematol. 84, 16–23 (1993).
Matthews, V. B. et al. Oncostatin M induces an acute phase response but does not modulate the growth or maturation-status of liver progenitor (oval) cells in culture. Exp. Cell Res. 306, 252–263 (2005).
Dierssen, U. et al. Molecular dissection of gp130-dependent pathways in hepatocytes during liver regeneration. J. Biol. Chem. 283, 9886–9895 (2008).
Alonzi, T. et al. Essential role of STAT3 in the control of the acute-phase response as revealed by inducible gene inactivation [correction of activation] in the liver. Mol. Cell Biol. 21, 1621–1632 (2001).
Kopf, M. et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994).
McFarland-Mancini, M. M. et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J. Immunol. 184, 7219–7228 (2010).
Weber, M. A. et al. Endogenous leukemia inhibitory factor attenuates endotoxin response. Lab. Invest. 85, 276–284 (2005).
Wallace, P. M. et al. Regulation of inflammatory responses by oncostatin M. J. Immunol. 162, 5547–5555 (1999).
Puel, A. et al. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J. Immunol. 180, 647–654 (2008).
Nanki, T. et al. Suppression of elevations in serum C reactive protein levels by anti-IL-6 autoantibodies in two patients with severe bacterial infections. Ann. Rheum. Dis. 72, 1100–1102 (2013).
Bloomfield, M. et al. Anti-IL6 autoantibodies in an infant with CRP-less septic shock. Front. Immunol. 10, 2629 (2019).
Shafran, I. H., Alasti, F., Smolen, J. S. & Aletaha, D. Implication of baseline levels and early changes of C-reactive protein for subsequent clinical outcomes of patients with rheumatoid arthritis treated with tocilizumab. Ann. Rheum. Dis. 79, 874–882 (2020).
Lee, J. Y. et al. Serum amyloid A proteins induce pathogenic Th17 cells and promote inflammatory disease. Cell 180, 79–91.e16 (2020).
Michalopoulos, G. K. & DeFrances, M. C. Liver regeneration. Science 276, 60–66 (1997).
Michalopoulos, G. K. Liver regeneration. J. Cell Physiol. 213, 286–300 (2007).
Fazel Modares, N. et al. IL-6 trans-signaling controls liver regeneration after partial hepatectomy. Hepatology 70, 2075–2091 (2019).
Drucker, C., Gewiese, J., Malchow, S., Scheller, J. & Rose-John, S. Impact of interleukin-6 classic- and trans-signaling on liver damage and regeneration. J. Autoimmun. 34, 29–37 (2010).
Haga, S. et al. Compensatory recovery of liver mass by Akt-mediated hepatocellular hypertrophy in liver-specific STAT3-deficient mice. J. Hepatol. 43, 799–807 (2005).
Riehle, K. J. et al. Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. J. Exp. Med. 205, 91–103 (2008).
Cressman, D. E. et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274, 1379–1383 (1996).
Blindenbacher, A. et al. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology 38, 674–682 (2003).
Fulop, A. K. et al. Hepatic regeneration induces transient acute phase reaction: systemic elevation of acute phase reactants and soluble cytokine receptors. Cell Biol. Int. 25, 585–592 (2001).
Peters, M. et al. Extramedullary expansion of hematopoietic progenitor cells in interleukin (IL)-6-sIL-6R double transgenic mice. J. Exp. Med. 185, 755–766 (1997).
Maione, D. et al. Coexpression of IL-6 and soluble IL-6R causes nodular regenerative hyperplasia and adenomas of the liver. EMBO J. 17, 5588–5597 (1998).
Nechemia-Arbely, Y. et al. Early hepatocyte DNA synthetic response posthepatectomy is modulated by IL-6 trans-signaling and PI3K/AKT activation. J. Hepatol. 54, 922–929 (2011).
Nakamura, K., Nonaka, H., Saito, H., Tanaka, M. & Miyajima, A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology 39, 635–644 (2004).
Okaya, A. et al. Oncostatin M inhibits proliferation of rat oval cells, OC15-5, inducing differentiation into hepatocytes. Am. J. Pathol. 166, 709–719 (2005).
Hamada, T. et al. Oncostatin M gene therapy attenuates liver damage induced by dimethylnitrosamine in rats. Am. J. Pathol. 171, 872–881 (2007).
Yang, Z. F. et al. Cardiotrophin-1 enhances regeneration of cirrhotic liver remnant after hepatectomy through promotion of angiogenesis and cell proliferation. Liver Int. 28, 622–631 (2008).
Omori, N. et al. Expression of leukemia inhibitory factor and its receptor during liver regeneration in the adult rat. Lab. Invest. 75, 15–24 (1996).
Rosenberg, D., Ilic, Z., Yin, L. & Sell, S. Proliferation of hepatic lineage cells of normal C57BL and interleukin-6 knockout mice after cocaine-induced periportal injury. Hepatology 31, 948–955 (2000).
Gajalakshmi, P. et al. Interleukin-6 secreted by bipotential murine oval liver stem cells induces apoptosis of activated hepatic stellate cells by activating NF-κB-inducible nitric oxide synthase signaling. Biochem. Cell Biol. 95, 263–272 (2017).
Fausto, N. & Campbell, J. S. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech. Dev. 120, 117–130 (2003).
Tirnitz-Parker, J. E. E., Forbes, S. J., Olynyk, J. K. & Ramm, G. A. Cellular plasticity in liver regeneration: spotlight on cholangiocytes. Hepatology 69, 2286–2289 (2019).
Nishina, T. et al. Interleukin-11 links oxidative stress and compensatory proliferation. Sci. Signal. 5, ra5 (2012).
Wahl, A. F. & Wallace, P. M. Oncostatin M in the anti-inflammatory response. Ann. Rheum. Dis. 60, iii75–iii80 (2001).
Zhu, C. et al. Hepatitis B virus enhances interleukin-27 expression both in vivo and in vitro. Clin. Immunol. 131, 92–97 (2009).
Katz, A., Chebath, J., Friedman, J. & Revel, M. Increased sensitivity of IL-6-deficient mice to carbon tetrachloride hepatotoxicity and protection with an IL-6 receptor-IL-6 chimera. Cytokines Cell Mol. Ther. 4, 221–227 (1998).
Klein, C. et al. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J. Clin. Invest. 115, 860–869 (2005).
Camargo, C. A. Jr., Madden, J. F., Gao, W., Selvan, R. S. & Clavien, P. A. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology 26, 1513–1520 (1997).
Hoge, J. et al. IL-6 controls the innate immune response against Listeria monocytogenes via classical IL-6 signaling. J. Immunol. 190, 703–711 (2013).
Jostock, T. et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 268, 160–167 (2001).
Barkhausen, T. et al. Selective blockade of interleukin-6 trans-signaling improves survival in a murine polymicrobial sepsis model. Crit. Care Med. 39, 1407–1413 (2011).
Li, S. Q., Zhu, S., Han, H. M., Lu, H. J. & Meng, H. Y. IL-6 trans-signaling plays important protective roles in acute liver injury induced by acetaminophen in mice. J. Biochem. Mol. Toxicol. 29, 288–297 (2015).
Gewiese-Rabsch, J., Drucker, C., Malchow, S., Scheller, J. & Rose-John, S. Role of IL-6 trans-signaling in CCl4 induced liver damage. Biochim. Biophys. Acta 1802, 1054–1061 (2010).
Zhao, J., Qi, Y. F. & Yu, Y. R. STAT3: a key regulator in liver fibrosis. Ann. Hepatol. 21, 100224 (2021).
Maeshima, K. et al. A protective role of interleukin 11 on hepatic injury in acute endotoxemia. Shock 21, 134–138 (2004).
Kawakami, T. et al. Highly liver-specific heme oxygenase-1 induction by interleukin-11 prevents carbon tetrachloride-induced hepatotoxicity. Int. J. Mol. Med. 18, 537–546 (2006).
Widjaja, A. A., Chothani, S. P. & Cook, S. A. Different roles of interleukin 6 and interleukin 11 in the liver: implications for therapy. Hum. Vaccin. Immunother. 16, 2357–2362 (2020).
Richards, C. D. The enigmatic cytokine oncostatin M and roles in disease. ISRN Inflamm. 2013, 512103 (2013).
Jones, G. W., Hill, D. G., Cardus, A. & Jones, S. A. IL-27: a double agent in the IL-6 family. Clin. Exp. Immunol. 193, 37–46 (2018).
Iñiguez, M. et al. Cardiotrophin-1 defends the liver against ischemia-reperfusion injury and mediates the protective effect of ischemic preconditioning. J. Exp. Med. 203, 2809–2815 (2006).
Tuñon, M. J. et al. Cardiotrophin-1 promotes a high survival rate in rabbits with lethal fulminant hepatitis of viral origin. J. Virol. 85, 13124–13132 (2011).
Sheng, T. et al. The relationship between serum interleukin-6 and the recurrence of hepatitis B virus related hepatocellular carcinoma after curative resection. Medicine 94, e941 (2015).
Naugler, W. E. et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317, 121–124 (2007).
Bosch, F. X., Ribes, J., Diaz, M. & Cleries, R. Primary liver cancer: worldwide incidence and trends. Gastroenterology 127, S5–S16 (2004).
Giannitrapani, L. et al. Circulating IL-6 and sIL-6R in patients with hepatocellular carcinoma. Ann. N. Y. Acad. Sci. 963, 46–52 (2002).
Bergmann, J. et al. IL-6 trans-signaling is essential for the development of hepatocellular carcinoma in mice. Hepatology 65, 89–103 (2017).
Rose-John, S. The soluble interleukin 6 receptor: advanced therapeutic options in inflammation. Clin. Pharmacol. Ther. 102, 591–598 (2017).
Xiang, Z. L., Zeng, Z. C., Fan, J., Tang, Z. Y. & Zeng, H. Y. Expression of connective tissue growth factor and interleukin-11 in intratumoral tissue is associated with poor survival after curative resection of hepatocellular carcinoma. Mol. Biol. Rep. 39, 6001–6006 (2012).
Zheng, H. et al. TMED3 promotes hepatocellular carcinoma progression via IL-11/STAT3 signaling. Sci. Rep. 6, 37070 (2016).
Widjaja, A. A. et al. Inhibiting interleukin 11 signaling reduces hepatocyte death and liver fibrosis, inflammation, and steatosis in mouse models of nonalcoholic steatohepatitis. Gastroenterology 157, 777–792.e14 (2019).
Hisaka, T. et al. Expression of leukemia inhibitory factor (LIF) and its receptor gp190 in human liver and in cultured human liver myofibroblasts. Cloning of new isoforms of LIF mRNA. Comp. Hepatol. 3, 10 (2004).
Santos, G. C. et al. Leukemia inhibitory factor (LIF) overexpression increases the angiogenic potential of bone marrow mesenchymal stem/stromal cells. Front. Cell Dev. Biol. 8, 778 (2020).
Ferrara, N., Winer, J. & Henzel, W. J. Pituitary follicular cells secrete an inhibitor of aortic endothelial cell growth: identification as leukemia inhibitory factor. Proc. Natl Acad. Sci. USA 89, 698–702 (1992).
Shi, Y. et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 569, 131–135 (2019).
Levy, M. T., Trojanowska, M. & Reuben, A. Oncostatin M: a cytokine upregulated in human cirrhosis, increases collagen production by human hepatic stellate cells. J. Hepatol. 32, 218–226 (2000).
Rebouissou, S. et al. Frequent in-frame somatic deletions activate gp130 in inflammatory hepatocellular tumours. Nature 457, 200–204 (2009).
Pilati, C. et al. Somatic mutations activating STAT3 in human inflammatory hepatocellular adenomas. J. Exp. Med. 208, 1359–1366 (2011).
Poussin, K. et al. Biochemical and functional analyses of gp130 mutants unveil JAK1 as a novel therapeutic target in human inflammatory hepatocellular adenoma. Oncoimmunology 2, e27090 (2013).
Okamura, Y. et al. Leukemia inhibitory factor receptor (LIFR) is detected as a novel suppressor gene of hepatocellular carcinoma using double-combination array. Cancer Lett. 289, 170–177 (2010).
Luo, Q. et al. Leukemia inhibitory factor receptor is a novel immunomarker in distinction of well-differentiated HCC from dysplastic nodules. Oncotarget 6, 6989–6999 (2015).
Ernst, M., Thiem, S., Nguyen, P. M., Eissmann, M. & Putoczki, T. L. Epithelial gp130/Stat3 functions: an intestinal signaling node in health and disease. Semin. Immunol. 26, 29–37 (2014).
Harris, P. R. et al. Recombinant Helicobacter pylori urease activates primary mucosal macrophages. J. Infect. Dis. 178, 1516–1520 (1998).
Sobala, G. M. et al. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut 32, 1415–1418 (1991).
Crabtree, J. E. Immune and inflammatory responses to Helicobacter pylori infection. Scand. J. Gastroenterol. Suppl. 215, 3–10 (1996).
Furukawa, K., Takahashi, T., Arai, F., Matsushima, K. & Asakura, H. Enhanced mucosal expression of interleukin-6 mRNA but not of interleukin-8 mRNA at the margin of gastric ulcer in Helicobacter pylori-positive gastritis. J. Gastroenterol. 33, 625–633 (1998).
Nishida, T. et al. Endothelin-1, an ulcer inducer, promotes gastric ulcer healing via mobilizing gastric myofibroblasts and stimulates production of stroma-derived factors. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G1041–G1050 (2006).
Pradeepkumar Singh, L., Kundu, P., Ganguly, K., Mishra, A. & Swarnakar, S. Novel role of famotidine in downregulation of matrix metalloproteinase-9 during protection of ethanol-induced acute gastric ulcer. Free Radic. Biol. Med. 43, 289–299 (2007).
Wen, C. Y. et al. Mechanism of the antiulcerogenic effect of IL-11 on acetic acid-induced gastric ulcer in rats. Life Sci. 70, 2997–3005 (2002).
Judd, L. M., Ulaganathan, M., Howlett, M. & Giraud, A. S. Cytokine signalling by gp130 regulates gastric mucosal healing after ulceration and, indirectly, antral tumour progression. J. Pathol. 217, 552–562 (2009).
Gallucci, R. M. et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 14, 2525–2531 (2000).
Ernst, M. et al. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J. Exp. Med. 194, 189–203 (2001).
de Souza, H. S. P., Fiocchi, C. & Iliopoulos, D. The IBD interactome: an integrated view of aetiology, pathogenesis and therapy. Nat. Rev. Gastroenterol. Hepatol. 14, 739–749 (2017).
Hosokawa, T. et al. Interleukin-6 and soluble interleukin-6 receptor in the colonic mucosa of inflammatory bowel disease. J. Gastroenterol. Hepatol. 14, 987–996 (1999).
Ganter, U., Arcone, R., Toniatti, C., Morrone, G. & Ciliberto, G. Dual control of C-reactive protein gene expression by interleukin-1 and interleukin-6. EMBO J. 8, 3773–3779 (1989).
Reinecker, H. C. et al. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1β by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn’s disease. Clin. Exp. Immunol. 94, 174–181 (1993).
Lovato, P. et al. Constitutive STAT3 activation in intestinal T cells from patients with Crohn’s disease. J. Biol. Chem. 278, 16777–16781 (2003).
Mudter, J. et al. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am. J. Gastroenterol. 100, 64–72 (2005).
Li, Y. et al. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut 59, 227–235 (2010).
Mitsuyama, K. et al. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut 36, 45–49 (1995).
Gustot, T. et al. Profile of soluble cytokine receptors in Crohn’s disease. Gut 54, 488–495 (2005).
Yamamoto, M., Yoshizaki, K., Kishimoto, T. & Ito, H. IL-6 is required for the development of Th1 cell-mediated murine colitis. J. Immunol. 164, 4878–4882 (2000).
Parisinos, C. A. et al. Variation in interleukin 6 receptor gene associates with risk of Crohn’s disease and ulcerative colitis. Gastroenterology 155, 303–306.e2 (2018).
Garbers, C. et al. The interleukin-6 receptor Asp358Ala single nucleotide polymorphism rs2228145 confers increased proteolytic conversion rates by ADAM proteases. Biochim. Biophys. Acta 1842, 1485–1494 (2014).
Scheller, J. & Rose-John, S. The interleukin 6 pathway and atherosclerosis. Lancet 380, 338 (2012).
Smillie, C. S. et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell 178, 714–730.e22 (2019).
Qiu, B. S., Pfeiffer, C. J. & Keith, J. C. Jr. Protection by recombinant human interleukin-11 against experimental TNB-induced colitis in rats. Dig. Dis. Sci. 41, 1625–1630 (1996).
Keith, J. C. Jr., Albert, L., Sonis, S. T., Pfeiffer, C. J. & Schaub, R. G. IL-11, a pleiotropic cytokine: exciting new effects of IL-11 on gastrointestinal mucosal biology. Stem Cell 12, 79–89; discussion 89-90 (1994).
Greenwood-Van Meerveld, B., Venkova, K. & Keith, J. C. Jr. Recombinant human interleukin-11 restores smooth muscle function in the jejunum and colon of human leukocyte antigen-B27 rats with intestinal inflammation. J. Pharmacol. Exp. Ther. 299, 58–66 (2001).
Gibson, D. L. et al. Interleukin-11 reduces TLR4-induced colitis in TLR2-deficient mice and restores intestinal STAT3 signaling. Gastroenterology 139, 1277–1288 (2010).
Lim, W. W. et al. Transgenic interleukin 11 expression causes cross-tissue fibro-inflammation and an inflammatory bowel phenotype in mice. PLoS ONE 15, e0227505 (2020).
Zhang, Y. S. et al. STAT4 activation by leukemia inhibitory factor confers a therapeutic effect on intestinal inflammation. EMBO J. 38, e99595 (2019).
Guimbaud, R. et al. Leukemia inhibitory factor involvement in human ulcerative colitis and its potential role in malignant course. Eur. Cytokine Netw. 9, 607–612 (1998).
Prieto-Vicente, V. et al. Cardiotrophin-1 attenuates experimental colitis in mice. Clin. Sci. 132, 985–1001 (2018).
Sanchez-Garrido, A. I. et al. Preventive effect of cardiotrophin-1 administration before DSS-induced ulcerative colitis in mice. J. Clin. Med. 8, 2086 (2019).
Beigel, F. et al. Oncostatin M mediates STAT3-dependent intestinal epithelial restitution via increased cell proliferation, decreased apoptosis and upregulation of SERPIN family members. PLoS ONE 9, e93498 (2014).
Sanchez, A. L. et al. Adenoviral transfer of the murine oncostatin M gene suppresses dextran-sodium sulfate-induced colitis. J. Interferon Cytokine Res. 23, 193–201 (2003).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
West, N. R. et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 23, 579–589 (2017).
Imielinski, M. et al. Common variants at five new loci associated with early-onset inflammatory bowel disease. Nat. Genet. 41, 1335–1340 (2009).
Wang, Z., Wang, L., Fan, R., Zhou, J. & Zhong, J. Association of IL-27 gene three polymorphisms with Crohn’s disease susceptibility in a Chinese Han population. Int. J. Clin. Exp. Pathol. 7, 8952–8957 (2014).
Li, C. S. et al. Interleukin-27 polymorphisms are associated with inflammatory bowel diseases in a Korean population. J. Gastroenterol. Hepatol. 24, 1692–1696 (2009).
Hanson, M. L. et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology 146, 210–221.e13 (2014).
Sasaoka, T. et al. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G568–G576 (2011).
Dambacher, J. et al. Interleukin 31 mediates MAP kinase and STAT1/3 activation in intestinal epithelial cells and its expression is upregulated in inflammatory bowel disease. Gut 56, 1257–1265 (2007).
Perrigoue, J. G., Zaph, C., Guild, K., Du, Y. & Artis, D. IL-31-IL-31R interactions limit the magnitude of Th2 cytokine-dependent immunity and inflammation following intestinal helminth infection. J. Immunol. 182, 6088–6094 (2009).
Nayar, S. et al. A myeloid-stromal niche and gp130 rescue in NOD2-driven Crohn’s disease. Nature 593, 275–281 (2021).
Putoczki, T. L. et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 24, 257–271 (2013).
Yoshizaki, A. et al. Expression of interleukin (IL)-11 and IL-11 receptor in human colorectal adenocarcinoma: IL-11 up-regulation of the invasive and proliferative activity of human colorectal carcinoma cells. Int. J. Oncol. 29, 869–876 (2006).
Wei, J. et al. Bazedoxifene as a novel GP130 inhibitor for colon cancer therapy. J. Exp. Clin. Cancer Res. 38, 63 (2019).
Thilakasiri, P. et al. Repurposing the selective estrogen receptor modulator bazedoxifene to suppress gastrointestinal cancer growth. EMBO Mol. Med. 11, e9539 (2019).
Lesina, M. et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell 19, 456–469 (2011).
Corcoran, R. B. et al. STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis. Cancer Res. 71, 5020–5029 (2011).
Zhang, Y. et al. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 73, 6359–6374 (2013).
Goumas, F. A. et al. Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int. J. Cancer 137, 1035–1046 (2015).
Wu, X., Cao, Y., Xiao, H., Li, C. & Lin, J. Bazedoxifene as a novel GP130 inhibitor for pancreatic cancer therapy. Mol. Cancer Ther. 15, 2609–2619 (2016).
Judd, L. M. et al. Gastric cancer development in mice lacking the SHP2 binding site on the IL-6 family co-receptor gp130. Gastroenterology 126, 196–207 (2004).
Jenkins, B. J. et al. Hyperactivation of Stat3 in gp130 mutant mice promotes gastric hyperproliferation and desensitizes TGF-β signaling. Nat. Med. 11, 845–852 (2005).
Tebbutt, N. C. et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat. Med. 8, 1089–1097 (2002).
Hill, D. G. et al. Hyperactive gp130/STAT3-driven gastric tumourigenesis promotes submucosal tertiary lymphoid structure development. Int. J. Cancer 143, 167–178 (2018).
Ernst, M. et al. STAT3 and STAT1 mediate IL-11-dependent and inflammation-associated gastric tumorigenesis in gp130 receptor mutant mice. J. Clin. Invest. 118, 1727–1738 (2008).
Jackson, C. B. et al. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J. Pathol. 213, 140–151 (2007).
Komoda, H. et al. Interleukin-6 levels in colorectal cancer tissues. World J. Surg. 22, 895–898 (1998).
Heikkila, K., Ebrahim, S. & Lawlor, D. A. Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur. J. Cancer 44, 937–945 (2008).
Grivennikov, S. et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 (2009).
Howlett, M. et al. The interleukin-6 family cytokine interleukin-11 regulates homeostatic epithelial cell turnover and promotes gastric tumor development. Gastroenterology 136, 967–977 (2009).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02641522 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02293837 (2021).
Wedell-Neergaard, A. S. et al. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: a randomized controlled trial. Cell Metab. 29, 844–855.e3 (2019).
US National Library of Medicine. ClinicalTrials.gov, https://clinicaltrials.gov/show/NCT01073826 (2016).
[No authors listed]. A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. ALS CNTF Treatment Study Group. Neurology 46, 1244-1249 (1996).
Ettinger, M. P. et al. Recombinant variant of ciliary neurotrophic factor for weight loss in obese adults: a randomized, dose-ranging study. Jama 289, 1826–1832 (2003).
Duff, E. & Baile, C. A. Ciliary neurotrophic factor: a role in obesity? Nutr. Rev. 61, 423–426 (2003).
Ito, H. et al. A pilot randomized trial of a human anti-interleukin-6 receptor monoclonal antibody in active Crohn’s disease. Gastroenterology 126, 989–996; discussion 947 (2004).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT01287897 (2016).
Danese, S. et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II). Gut 68, 40–48 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT01545050 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT01635621 (2012).
Mitsuyama, K. et al. STAT3 activation via interleukin 6 trans-signalling contributes to ileitis in SAMP1/Yit mice. Gut 55, 1263–1269 (2006).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat. Med. 6, 583–588 (2000).
Schreiber, S. et al. Therapeutic interleukin 6 trans-signaling inhibition by olamkicept (sgp130Fc) in patients with active inflammatory bowel disease. Gastroenterology https://doi.org/10.1053/j.gastro.2021.02.062 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03235752 (2021).
Sands, B. E. et al. Preliminary evaluation of safety and activity of recombinant human interleukin 11 in patients with active Crohn’s disease. Gastroenterology 117, 58–64 (1999).
Sands, B. E. et al. Randomized, controlled trial of recombinant human interleukin-11 in patients with active Crohn’s disease. Aliment. Pharmacol. Ther. 16, 399–406 (2002).
Herrlinger, K. R. et al. Randomized, double blind controlled trial of subcutaneous recombinant human interleukin-11 versus prednisolone in active Crohn’s disease. Am. J. Gastroenterol. 101, 793–797 (2006).
Sandborn, W. J. et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 367, 616–624 (2012).
Sandborn, W. J. et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376, 1723–1736 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03281304 (2021).
Panes, J. et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 66, 1049–1059 (2017).
Sands, B. E. et al. Peficitinib, an oral janus kinase inhibitor, in moderate-to-severe ulcerative colitis: results from a randomised, phase 2 study. J. Crohns Colitis 12, 1158–1169 (2018).
Vermeire, S. et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 389, 266–275 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02914600 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02914535 (2021).
Sandborn, W. J. et al. Efficacy and safety of upadacitinib in a randomized trial of patients with Crohn’s disease. Gastroenterology 158, 2123–2138.e8 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03006068 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03345836 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03675477 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03677648 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03635112 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03920254 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03395184 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02958865 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT04353791 (2021).
Wilde, M. I. & Faulds, D. Oprelvekin: a review of its pharmacology and therapeutic potential in chemotherapy-induced thrombocytopenia. BioDrugs 10, 159–171 (1998).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03490669 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT04191421 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03382340 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02119676 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02277093 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03522649 (2019).
Okusaka, T. et al. Phase 1 and pharmacological trial of OPB-31121, a signal transducer and activator of transcription-3 inhibitor, in patients with advanced hepatocellular carcinoma. Hepatol. Res. 45, 1283–1291 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT01839604 (2017).
Plimack, E. R. et al. AZD1480: a phase I study of a novel JAK2 inhibitor in solid tumors. Oncologist 18, 819–820 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT01219543 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT04358185 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT04374877 (2020).
Spencer, S. et al. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J. Exp. Med. 216, 1986–1998 (2019).
Chen, Y. H. et al. Absence of GP130 cytokine receptor signaling causes extended Stüve-Wiedemann syndrome. J. Exp. Med. 217, e20191306 (2020).
Schwerd, T. et al. A biallelic mutation in IL6ST encoding the GP130 co-receptor causes immunodeficiency and craniosynostosis. J. Exp. Med. 214, 2547–2562 (2017).
Shahin, T. et al. Selective loss of function variants in IL6ST cause hyper-IgE syndrome with distinct impairments of T-cell phenotype and function. Haematologica 104, 609–621 (2019).
Minegishi, Y. et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity 25, 745–755 (2006).
Boisson-Dupuis, S. et al. Tuberculosis and impaired IL-23-dependent IFN-γ immunity in humans homozygous for a common TYK2 missense variant. Sci. Immunol. 3, eaau8714 (2018).
Minegishi, Y. et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature 448, 1058–1062 (2007).
Fabre, A. et al. Clinical aspects of STAT3 gain-of-function germline mutations: a systematic review. J. Allergy Clin. Immunol. Pract. 7, 1958–1969.e9 (2019).
Yang, S. et al. Activating JAK1 mutation may predict the sensitivity of JAK-STAT inhibition in hepatocellular carcinoma. Oncotarget 7, 5461–5469 (2016).
Ungureanu, D. et al. The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling. Nat. Struct. Mol. Biol. 18, 971–976 (2011).
Keupp, K. et al. Mutations in the interleukin receptor IL11RA cause autosomal recessive Crouzon-like craniosynostosis. Mol. Genet. Genomic Med. 1, 223–237 (2013).
Brischoux-Boucher, E. et al. IL11RA-related Crouzon-like autosomal recessive craniosynostosis in 10 new patients: resemblances and differences. Clin. Genet. 94, 373–380 (2018).
Arita, K. et al. Oncostatin M receptor-β mutations underlie familial primary localized cutaneous amyloidosis. Am. J. Hum. Genet. 82, 73–80 (2008).
Dagoneau, N. et al. Null leukemia inhibitory factor receptor (LIFR) mutations in Stuve-Wiedemann/Schwartz-Jampel type 2 syndrome. Am. J. Hum. Genet. 74, 298–305 (2004).
Acknowledgements
D.C. is supported by a predoctoral iPFIS (IFI 19/00048) funded by the Spanish Institute of Health Carlos III (co-funded by the European Social Fund). M.D.G. acknowledges support from a Juan Rodes contract (JR18/00026) funded by the Spanish Institute of Health Carlos III (co-funded by the European Social Fund). This study is supported by MINECO/AEI/FEDER, UE PID2019-110587RB-I00 from the Ministry of Economy and Competitiveness (co-funded by the European Social Fund) and Andalusian Ministry of Economy, Innovation, Science and Employment (P18-RT-4775). S.R.- J. is funded by the German Research Foundation (DFG, project number 80750187 – SFB 841 (project C1). S.R.- J. and C.G. are funded by the German Research Foundation (DFG, project number 125440785 – SFB 877 (projects A1, A10 and A14)).
Author information
Authors and Affiliations
Contributions
All the authors contributed to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
S.R.-J. has acted as a consultant and speaker for AbbVie, Chugai, Genentech Roche, Pfizer and Sanofi. He also declares that he is an inventor on patents owned by CONARIS Research Institute, which develops the sgp130Fc protein olamkicept together with the company I-Mab. S.R.-J. has stock ownership in CONARIS. C.G. has received a research grant from Corvidia Therapeutics (Waltham, MA, USA) and has acted as a consultant for AbbVie. All other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks M. Ernst, T. Kishimoto and H. Uhlig for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Giraldez, M.D., Carneros, D., Garbers, C. et al. New insights into IL-6 family cytokines in metabolism, hepatology and gastroenterology. Nat Rev Gastroenterol Hepatol 18, 787–803 (2021). https://doi.org/10.1038/s41575-021-00473-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-021-00473-x
This article is cited by
-
Structural insights into IL-11-mediated signalling and human IL6ST variant-associated immunodeficiency
Nature Communications (2024)
-
Suppression of cytokine release syndrome during CAR-T-cell therapy via a subcutaneously injected interleukin-6-adsorbing hydrogel
Nature Biomedical Engineering (2023)
-
Cytokimera GIL-11 rescued IL-6R deficient mice from partial hepatectomy-induced death by signaling via non-natural gp130:LIFR:IL-11R complexes
Communications Biology (2023)
-
The immunological role of ADAMs in the field of gastroenterological chronic inflammatory diseases and cancers: a review
Oncogene (2023)
-
Targeting IL-6 trans-signalling: past, present and future prospects
Nature Reviews Immunology (2023)