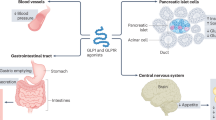
Abstract
Glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide 1 (GLP1) exhibit incretin activity, meaning that they potentiate glucose-dependent insulin secretion. The emergence of GIP receptor (GIPR)–GLP1 receptor (GLP1R) co-agonists has fostered growing interest in the actions of GIP and GLP1 in metabolically relevant tissues. Here, we update concepts of how these hormones act beyond the pancreas. The actions of GIP and GLP1 on liver, muscle and adipose tissue, in the control of glucose and lipid homeostasis, are discussed in the context of plausible mechanisms of action. Both the GIPR and GLP1R are expressed in the central nervous system, wherein receptor activation produces anorectic effects enabling weight loss. In preclinical studies, GIP and GLP1 reduce atherosclerosis. Furthermore, GIPR and GLP1R are expressed within the heart and immune system, and GLP1R within the kidney, revealing putative mechanisms linking GIP and GLP1R agonism to cardiorenal protection. We interpret the clinical and mechanistic data obtained for different agents that enable weight loss and glucose control for the treatment of obesity and type 2 diabetes mellitus, respectively, by activating or blocking GIPR signalling, including the GIPR–GLP1R co-agonist tirzepatide, as well as the GIPR antagonist–GLP1R agonist AMG-133. Collectively, we update translational concepts of GIP and GLP1 action, while highlighting gaps, areas of uncertainty and controversies meriting ongoing investigation.
Key points
-
Glucagon-like peptide 1 (GLP1) receptor and glucose-dependent insulinotropic polypeptide (GIP) receptor are widely expressed in multiple organs beyond the pancreas.
-
GIP and GLP1 reduce appetite by signalling through their receptors that are expressed in multiple regions of the central nervous system.
-
GIP suppresses macrophage-dependent inflammation, whereas GLP1 reduces gut inflammation through its receptor on intraepithelial lymphocytes.
-
Both GLP1 and GIP act indirectly on white adipose tissue, whereas GIP directly regulates fat and amino acid metabolism and inflammation within brown adipose tissue.
-
GIP and GLP1 are neuroprotective in preclinical models of neurodegenerative disease.
-
Current insight into the cardiovascular biology of GIP is limited, whereas GLP1 reduces major adverse cardiovascular events in humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
McIntyre, N., Holdsworth, C. D. & Turner, D. S. Intestinal factors in the control of insulin secretion. J. Clin. Endocrinol. Metab. 25, 1317–1324 (1965).
Brown, J. C. & Dryburgh, J. R. A gastric inhibitory polypeptide. II. The complete amino acid sequence. Can. J. Biochem. 49, 867–872 (1971).
Jorsal, T. et al. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia 61, 284–294 (2018).
Ugleholdt, R. et al. Prohormone convertase 1/3 is essential for processing of the glucose-dependent insulinotropic polypeptide precursor. J. Biol. Chem. 281, 11050–11057 (2006).
Drucker, D. J., Habener, J. F. & Holst, J. J. Discovery, characterization, and clinical development of the glucagon-like peptides. J. Clin. Invest. 127, 4217–4227 (2017).
Song, Y. et al. Gut-proglucagon-derived peptides are essential for regulating glucose homeostasis in mice. Cell Metab. 30, 976–986.e3 (2019).
Mayo, K. E. et al. International union of pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev. 55, 167–194 (2003).
El, K. et al. GIP mediates the incretin effect and glucose tolerance by dual actions on alpha cells and beta cells. Sci. Adv. 7, eabf1948 (2021).
Segerstolpe, A. et al. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 24, 593–607 (2016).
Ast, J. et al. Super-resolution microscopy compatible fluorescent probes reveal endogenous glucagon-like peptide-1 receptor distribution and dynamics. Nat. Commun. 11, 467 (2020).
Panjwani, N. et al. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE−/− mice. Endocrinology 154, 127–139 (2013).
Ussher, J. R. et al. Inactivation of the glucose-dependent insulinotropic polypeptide receptor improves outcomes following experimental myocardial infarction. Cell Metab. 27, 450–460 (2018).
Ast, J., Broichhagen, J. & Hodson, D. J. Reagents and models for detecting endogenous GLP1R and GIPR. eBioMedicine 74, 103739 (2021).
Gray, S. M. et al. Discordance between GLP-1R gene and protein expression in mouse pancreatic islet cells. J. Biol. Chem. 295, 11529–11541 (2020).
Usdin, T. B., Mezey, E., Button, D. C., Brownstein, M. J. & Bonner, T. I. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology 133, 2861–2870 (1993).
Campos, R. V., Lee, Y. C. & Drucker, D. J. Divergent tissue-specific and developmental expression of receptors for glucagon and glucagon-like peptide-1 in the mouse. Endocrinology 134, 2156–2164 (1994).
Dunphy, J. L., Taylor, R. G. & Fuller, P. J. Tissue distribution of rat glucagon receptor and GLP-1 receptor gene expression. Mol. Cell Endocrinol. 141, 179–186 (1998).
Christensen, M. B., Calanna, S., Holst, J. J., Vilsboll, T. & Knop, F. K. Glucose-dependent insulinotropic polypeptide: blood glucose stabilizing effects in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 99, E418–E426 (2014).
Campbell, J. E. & Drucker, D. J. Pharmacology physiology and mechanisms of incretin hormone action. Cell Metab. 17, 819–837 (2013).
Mentlein, R., Gallwitz, B. & Schmidt, W. E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 214, 829–835 (1993).
Kieffer, T. J., McIntosh, C. H. & Pederson, R. A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 136, 3585–3596 (1995).
Hojberg, P. V. et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 52, 199–207 (2009).
Drucker, D. J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 27, 740–756 (2018).
Pi-Sunyer, X. et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N. Engl. J. Med. 373, 11–22 (2015).
Wilding, J. P. H. et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 384, 989 (2021).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 9, 653–662 (2021).
Baggio, L. L. & Drucker, D. J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol. Metab. 46, 101090 (2021).
McLean, B. A. et al. Revisiting the complexity of GLP-1 action from sites of synthesis to receptor activation. Endocr. Rev. 42, 101–132 (2021).
Wang, N. et al. Vasodilatory actions of glucagon-like peptide 1 are preserved in skeletal and cardiac muscle microvasculature but not in conduit artery in obese humans with vascular insulin resistance. Diabetes Care 43, 634–642 (2020).
Asmar, M. et al. Glucose-dependent insulinotropic polypeptide has impaired effect on abdominal, subcutaneous adipose tissue metabolism in obese subjects. Int. J. Obes. 38, 259–265 (2014).
Snook, L. A., Nelson, E. M., Dyck, D. J., Wright, D. C. & Holloway, G. P. Glucose-dependent insulinotropic polypeptide directly induces glucose transport in rat skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R295–R303 (2015).
Bertin, E., Arner, P., Bolinder, J. & Hagstrom-Toft, E. Action of glucagon and glucagon-like peptide-1-(7–36) amide on lipolysis in human subcutaneous adipose tissue and skeletal muscle in vivo. J. Clin. Endocrinol. Metab. 86, 1229–1234 (2001).
Chai, W. et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 61, 888–896 (2012).
Subaran, S. C. et al. GLP-1 at physiological concentrations recruits skeletal and cardiac muscle microvasculature in healthy humans. Clin. Sci. 127, 163–170 (2014).
Heimburger, S. M. N. et al. GIP affects hepatic fat and brown adipose tissue thermogenesis, but not white adipose tissue transcriptome in T1D. J. Clin. Endocrinol. Metab. https://doi.org/10.1210/clinem/dgac542 (2022).
Miyawaki, K. et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med. 8, 738–742 (2002).
Althage, M. C. et al. Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. J. Biol. Chem. 283, 18365–18376 (2008).
Killion, E. A. et al. Anti-obesity effects of GIPR antagonists alone and in combination with GLP-1R agonists in preclinical models. Sci. Transl. Med. 10, eaat3392 (2018).
Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med. 384, 1113–1124 (2021).
Landgraf, D. et al. Oxyntomodulin regulates resetting of the liver circadian clock by food. eLife 4, e06253 (2015).
McLean, B. A., Wong, C. K., Kaur, K. D., Seeley, R. J. & Drucker, D. J. Differential importance of endothelial and hematopoietic cell GLP-1Rs for cardiometabolic versus hepatic actions of semaglutide. JCI Insight 6, e153732 (2021).
Asmar, M. et al. The gluco- and liporegulatory and vasodilatory effects of glucose-dependent insulinotropic polypeptide (GIP) are abolished by an antagonist of the human GIP receptor. Diabetes 66, 2363–2371 (2017).
Saari, T. et al. Obesity-associated blunted subcutaneous adipose tissue blood flow after meal improves after bariatric surgery. J. Clin. Endocrinol. Metab. 107, 1930–1938 (2022).
Thondam, S. K. et al. Glucose-dependent insulinotropic polypeptide promotes lipid deposition in subcutaneous adipocytes in obese type 2 diabetes patients: a maladaptive response. Am. J. Physiol. Endocrinol. Metab. 312, E224–E233 (2017).
Kim, S. J., Nian, C. & McIntosh, C. H. GIP increases human adipocyte LPL expression through CREB and TORC2-mediated trans-activation of the LPL gene. J. Lipid Res. 51, 3145–3157 (2010).
Getty-Kaushik, L., Song, D. H., Boylan, M. O., Corkey, B. E. & Wolfe, M. M. Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity 14, 1124–1131 (2006).
Hansotia, T. et al. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J. Clin. Invest. 117, 143–152 (2007).
Svendsen, B. et al. Pharmacological antagonism of the incretin system protects against diet-induced obesity. Mol. Metab. 32, 44–55 (2020).
Killion, E. A. et al. Chronic glucose-dependent insulinotropic polypeptide receptor (GIPR) agonism desensitizes adipocyte GIPR activity mimicking functional GIPR antagonism. Nat. Commun. 11, 4981 (2020).
Joo, E. et al. Inhibition of gastric inhibitory polypeptide receptor signaling in adipose tissue reduces insulin resistance and hepatic steatosis in high-fat diet-fed mice. Diabetes 66, 868–879 (2017).
Campbell, J. E. et al. GIPR is predominantly localized to nonadipocyte cell types within white adipose tissue. Diabetes 71, 1115–1127 (2022).
Beaudry, J. L. et al. Physiological roles of the GIP receptor in murine brown adipose tissue. Mol. Metab. 28, 14–25 (2019).
Samms, R. J. et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. J. Clin. Invest. 131, e146353 (2021).
Samms, R. J. et al. Tirzepatide induces a thermogenic-like amino acid signature in brown adipose tissue. Mol. Metab. 64, 101550 (2022).
Beiroa, D. et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 63, 3346–3358 (2014).
van Can, J. et al. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. 38, 784–793 (2014).
Bollag, R. J. et al. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology 141, 1228–1235 (2000).
Zhong, Q. et al. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am. J. Physiol. Endocrinol. Metab. 292, E543–E548 (2007).
Christensen, M. B. et al. Glucose-dependent insulinotropic polypeptide (GIP) inhibits bone resorption independently of insulin and glycemia. J. Clin. Endocrinol. Metab. 103, 288–294 (2018).
Torekov, S. S. et al. A functional amino acid substitution in the glucose-dependent insulinotropic polypeptide receptor (GIPR) gene is associated with lower bone mineral density and increased fracture risk. J. Clin. Endocrinol. Metab. 99, E729–E733 (2014).
Bjerre Knudsen, L. et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 151, 1473–1486 (2010).
Yamada, C. et al. The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology 149, 574–579 (2008).
Patorno, E. et al. Comparative effectiveness and safety of sodium-glucose cotransporter 2 inhibitors versus glucagon-like peptide 1 receptor agonists in older adults. Diabetes Care 44, 826–835 (2021).
Mantelmacher, F. D. et al. Glucose-dependent insulinotropic polypeptide receptor deficiency leads to impaired bone marrow hematopoiesis. J. Immunol. 198, 3089–3098 (2017).
Pujadas, G. et al. The gut hormone receptor GIPR links energy availability to the control of hematopoiesis. Mol. Metab. 39, 101008 (2020).
Mantelmacher, F. D. et al. GIP regulates inflammation and body weight by restraining myeloid-cell-derived S100A8/A9. Nat. Metab. 1, 58–69 (2019).
Varol, C. et al. Long-acting glucose-dependent insulinotropic polypeptide ameliorates obesity-induced adipose tissue inflammation. J. Immunol. 193, 4002–4009 (2014).
Kim, S. J. et al. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS ONE 7, e40156 (2012).
Chen, S., Okahara, F., Osaki, N. & Shimotoyodome, A. Increased GIP signaling induces adipose inflammation via a HIF-1alpha-dependent pathway and impairs insulin sensitivity in mice. Am. J. Physiol. Endocrinol. Metab. 308, E414–E425 (2015).
Gogebakan, O. et al. GIP increases adipose tissue expression and blood levels of MCP-1 in humans and links high energy diets to inflammation: a randomised trial. Diabetologia 58, 1759–1768 (2015).
Yusta, B. et al. GLP-1 receptor (GLP-1R) agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte (IEL) GLP-1R. Diabetes 64, 2537–2549 (2015).
Kahles, F. et al. GLP-1 secretion is increased by inflammatory stimuli in an IL-6-dependent manner, leading to hyperinsulinemia and blood glucose lowering. Diabetes 63, 3221–3229 (2014).
Ellingsgaard, H. et al. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat. Med. 17, 1481–1489 (2011).
Nguyen, A. T. et al. Lipopolysaccharides-mediated increase in glucose-stimulated insulin secretion: involvement of the GLP-1 pathway. Diabetes 63, 471–482 (2014).
Lebrun, L. J. et al. Enteroendocrine L cells sense LPS after gut barrier injury to enhance GLP-1 secretion. Cell Rep. 21, 1160–1168 (2017).
Kahles, F. et al. Glucagon-like peptide 1 levels predict cardiovascular risk in patients with acute myocardial infarction. Eur. Heart J. 41, 882–889 (2020).
Brakenridge, S. C. et al. Persistently elevated glucagon-like peptide-1 levels among critically ill surgical patients after sepsis and development of chronic critical illness and dismal long-term outcomes. J. Am. Coll. Surg. 229, 58–67.e1 (2019).
Lebherz, C. et al. GLP-1 levels predict mortality in patients with critical illness as well as end-stage renal disease. Am. J. Med. 130, 833–841.e3 (2017).
Noyan-Ashraf, M. H. et al. A glucagon-like peptide-1 analogue reverses the molecular pathology and cardiac dysfunction of a mouse model of obesity. Circulation 127, 74–85 (2013).
Wong, C. K. et al. Divergent roles for the gut intraepithelial lymphocyte GLP-1R in control of metabolism, microbiota, and T cell-induced inflammation. Cell Metab. 34, 1514–1531.e7 (2022).
Lee, Y. S. et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia 55, 2456–2468 (2012).
Chaudhuri, A. et al. Exenatide exerts a potent antiinflammatory effect. J. Clin. Endocrinol. Metab. 97, 198–207 (2012).
Rodbard, H. W. et al. Oral semaglutide versus empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the PIONEER 2 trial. Diabetes Care 42, 2272–2281 (2019).
Hadjiyanni, I., Siminovitch, K. A., Danska, J. S. & Drucker, D. J. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia 53, 730–740 (2010).
He, S. et al. Gut intraepithelial T cells calibrate metabolism and accelerate cardiovascular disease. Nature 566, 115–119 (2019).
Yoon, H. S. et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 6, 563–573 (2021).
Chaudhari, S. N. et al. A microbial metabolite remodels the gut-liver axis following bariatric surgery. Cell Host Microbe 29, 408–424.e7 (2021).
Grasset, E. et al. A specific gut microbiota dysbiosis of type 2 diabetic mice induces GLP-1 resistance through an enteric NO-dependent and gut-brain axis mechanism. Cell Metab. 25, 1075–1090.e5 (2017).
Martchenko, S. E. et al. Circadian GLP-1 secretion in mice is dependent on the intestinal microbiome for maintenance of diurnal metabolic homeostasis. Diabetes 69, 2589–2602 (2020).
Heiss, C. N. et al. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 35, 109163 (2021).
Smits, M. M. et al. Liraglutide and sitagliptin have no effect on intestinal microbiota composition: a 12-week randomized placebo-controlled trial in adults with type 2 diabetes. Diabetes Metab. 47, 101223 (2021).
Somm, E. et al. The GLP-1R agonist liraglutide limits hepatic lipotoxicity and inflammatory response in mice fed a methionine-choline deficient diet. Transl. Res. 227, 75–88 (2021).
Trevaskis, J. L. et al. Glucagon-like peptide-1 receptor agonism improves metabolic, biochemical, and histopathological indices of nonalcoholic steatohepatitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G762–G772 (2012).
Armstrong, M. J. et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 387, 679–690 (2016).
Meier, J. J. et al. Secretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjects. Diabetes 53, 654–662 (2004).
Idorn, T. et al. Elimination and degradation of glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with end-stage renal disease. J. Clin. Endocrinol. Metab. 99, 2457–2466 (2014).
Pyke, C. et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155, 1280–1290 (2014).
Jensen, E. P. et al. Activation of GLP-1 receptors on vascular smooth muscle cells reduces the autoregulatory response in afferent arterioles and increases renal blood flow. Am. J. Physiol. Ren. Physiol. 308, F867–F877 (2015).
Rieg, T. et al. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am. J. Physiol. Ren. Physiol. 303, F963–F971 (2012).
Gutzwiller, J. P. et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J. Clin. Endocrinol. Metab. 89, 3055–3061 (2004).
Lovshin, J. A. et al. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care 38, 132–139 (2015).
Fujita, H. et al. The protective roles of GLP-1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int. 85, 579–589 (2014).
Ougaard, M. E. et al. Liraglutide improves the kidney function in a murine model of chronic kidney disease. Nephron 144, 595–606 (2020).
Moschovaki Filippidou, F. et al. Glucagon-like peptide-1 receptor agonism improves nephrotoxic serum nephritis by inhibiting T-cell proliferation. Am. J. Pathol. 190, 400–411 (2020).
Leehey, D. J., Rahman, M. A., Borys, E., Picken, M. M. & Clise, C. E. Acute kidney injury associated with semaglutide. Kidney Med. 3, 282–285 (2021).
Chen, J. J. et al. Association of glucagon-like peptide-1 receptor agonist vs dipeptidyl peptidase-4 inhibitor use with mortality among patients with type 2 diabetes and advanced chronic kidney disease. JAMA Netw. Open 5, e221169 (2022).
Kristensen, S. L. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 7, 776–785 (2019).
Gerstein, H. C. et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet 394, 131–138 (2019).
Shaman, A. M. et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: pooled analysis of SUSTAIN 6 and LEADER. Circulation 145, 575–585 (2022).
Pasternak, B. et al. Use of glucagon-like peptide 1 receptor agonists and risk of serious renal events: Scandinavian cohort study. Diabetes Care 43, 1326–1335 (2020).
Baggio, L. L. et al. GLP-1 receptor expression within the human heart. Endocrinology 159, 1570–1584 (2018).
Jujic, A. et al. Glucose-dependent insulinotropic peptide in the high-normal range is associated with increased carotid intima–media thickness. Diabetes Care 44, 224–230 (2021).
Bowker, N. et al. Genetically predicted glucose-dependent insulinotropic polypeptide (GIP) levels and cardiovascular disease risk are driven by distinct causal variants in the GIPR region. Diabetes 70, 2706–2719 (2021).
Nogi, Y. et al. Glucose-dependent insulinotropic polypeptide prevents the progression of macrophage-driven atherosclerosis in diabetic apolipoprotein E-null mice. PLoS ONE 7, e35683 (2012).
Pujadas, G. et al. Genetic disruption of the Gipr in Apoe(−/−) mice promotes atherosclerosis. Mol. Metab. 65, 101586 (2022).
Karhunen, V. et al. Leveraging human genetic data to investigate the cardiometabolic effects of glucose-dependent insulinotropic polypeptide signalling. Diabetologia 64, 2773–2778 (2021).
Terasaki, M. et al. Glucose-dependent insulinotropic polypeptide suppresses foam cell formation of macrophages through inhibition of the cyclin-dependent kinase 5–CD36 pathway. Biomedicines 9, 832 (2021).
Kahles, F. et al. The incretin hormone GIP is upregulated in patients with atherosclerosis and stabilizes plaques in ApoE(−/−) mice by blocking monocyte/macrophage activation. Mol. Metab. 14, 150–157 (2018).
Mori, Y. et al. Glucose-dependent insulinotropic polypeptide suppresses peripheral arterial remodeling in male mice. Endocrinology 159, 2717–2732 (2018).
Hiromura, M. et al. Suppressive effects of glucose-dependent insulinotropic polypeptide on cardiac hypertrophy and fibrosis in angiotensin II-infused mouse models. Circ. J. 80, 1988–1997 (2016).
Drucker, D. J. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 24, 15–30 (2016).
Cahill, K. N. et al. Glucagon-like peptide-1 receptor regulates thromboxane-induced human platelet activation. JACC Basic Transl. Sci. 7, 713–715 (2022).
Varin, E. M. et al. Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action. Cell Rep. 27, 3371–3384.e3 (2019).
Noyan-Ashraf, M. H. et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 58, 975–983 (2009).
Mclean, B. A., Wong, C. K., Kabir, M. G. & Drucker, D. J. Glucagon-like peptide-1 receptor Tie2+ cells are essential for the cardioprotective actions of liraglutide in mice with experimental myocardial infarction. Mol. Metab. https://doi.org/10.1016/j.molmet.2022.101641 (2022).
Ussher, J. R. et al. Inactivation of the cardiomyocyte glucagon-like peptide-1 receptor (GLP-1R) unmasks cardiomyocyte-independent GLP-1R-mediated cardioprotection. Mol. Metab. 3, 507–517 (2014).
Nagashima, M. et al. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein E knockout mice. Diabetologia 54, 2649–2659 (2011).
Rakipovski, G. et al. The GLP-1 analogs liraglutide and semaglutide reduce atherosclerosis in ApoE(−/−) and LDLr(−/−) mice by a mechanism that includes inflammatory pathways. JACC Basic Transl. Sci. 3, 844–857 (2018).
Helmstadter, J. et al. Endothelial GLP-1 (glucagon-like peptide-1) receptor mediates cardiovascular protection by liraglutide in mice with experimental arterial hypertension. Arterioscler. Thromb. Vasc. Biol. 40, 145–158 (2020).
Cherney, D. Z. I., Udell, J. A. & Drucker, D. J. Cardiorenal mechanisms of action of glucagon-like-peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors. Med (N Y) 2, 1203–1230 (2021).
Gerstein, H. C. et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N. Engl. J. Med. 385, 896–907 (2021).
Dave, C. V. et al. Risk of cardiovascular outcomes in patients with type 2 diabetes after addition of SGLT2 inhibitors versus sulfonylureas to baseline GLP-1RA therapy. Circulation 143, 770–779 (2021).
Ryan, D. H. et al. Semaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am. Heart J. 229, 61–69 (2020).
Ripa, R. S. et al. Effect of liraglutide on arterial inflammation assessed as [(18)F]FDG uptake in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Circ. Cardiovasc. Imaging 14, e012174 (2021).
Jensen, J. K. et al. Effect of 26 weeks of liraglutide treatment on coronary artery inflammation in type 2 diabetes quantified by [(64)Cu]Cu-DOTATATE PET/CT: results from the LIRAFLAME trial. Front. Endocrinol. 12, 790405 (2021).
Koska, J., Migrino, R. Q., Chan, K. C., Cooper-Cox, K. & Reaven, P. D. The effect of exenatide once weekly on carotid atherosclerosis in individuals with type 2 diabetes: an 18-month randomized placebo-controlled study. Diabetes Care 44, 1385–1392 (2021).
Zobel, E. H. et al. Effect of liraglutide on expression of inflammatory genes in type 2 diabetes. Sci. Rep. 11, 18522 (2021).
Nyberg, J. et al. Glucose-dependent insulinotropic polypeptide is expressed in adult hippocampus and induces progenitor cell proliferation. J. Neurosci. 25, 1816–1825 (2005).
Nyberg, J., Jacobsson, C., Anderson, M. F. & Eriksson, P. S. Immunohistochemical distribution of glucose-dependent insulinotropic polypeptide in the adult rat brain. J. Neurosci. Res. 85, 2099–2119 (2007).
Adriaenssens, A. E. et al. Glucose-dependent insulinotropic polypeptide receptor-expressing cells in the hypothalamus regulate food intake. Cell Metab. 30, 987–996.e6 (2019).
Smith, C. et al. A comparative transcriptomic analysis of glucagon-like peptide-1 receptor- and glucose-dependent insulinotropic polypeptide-expressing cells in the hypothalamus. Appetite 174, 106022 (2022).
Ludwig, M. Q. et al. A genetic map of the mouse dorsal vagal complex and its role in obesity. Nat. Metab. 3, 530–545 (2021).
Bates, H. E. et al. Gipr is essential for adrenocortical steroidogenesis; however, corticosterone deficiency does not mediate the favorable metabolic phenotype of Gipr(−/−) mice. Diabetes 61, 40–48 (2012).
Kaneko, K. et al. Gut-derived GIP activates central Rap1 to impair neural leptin sensitivity during overnutrition. J. Clin. Invest. 130, 3786–3791 (2019).
Zhang, Q. et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS-GIPR signaling. Cell Metab. 33, 833–844.e5 (2021).
Drucker, D. J. GLP-1 physiology informs the pharmacotherapy of obesity. Mol. Metab. 57, 101351 (2022).
Zhang, T., Perkins, M. H., Chang, H., Han, W. & de Araujo, I. E. An inter-organ neural circuit for appetite suppression. Cell 185, 2478–2494.e28 (2022).
Nauck, M. A., Kemmeries, G., Holst, J. J. & Meier, J. J. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes 60, 1561–1565 (2011).
Plamboeck, A. et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G1117–G1127 (2013).
Gabery, S. et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 5, e133429 (2020).
Burmeister, M. A. et al. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes 66, 372–384 (2017).
Fortin, S. M. et al. GABA neurons in the nucleus tractus solitarius express GLP-1 receptors and mediate anorectic effects of liraglutide in rats. Sci. Transl. Med. 12, eaay8071 (2020).
Duffy, A. M. & Holscher, C. The incretin analogue D-Ala(2)GIP reduces plaque load, astrogliosis and oxidative stress in an APP/PS1 mouse model of Alzheimer’s disease. Neuroscience 228, 294–300 (2013).
Faivre, E., Hamilton, A. & Holscher, C. Effects of acute and chronic administration of GIP analogues on cognition, synaptic plasticity and neurogenesis in mice. Eur. J. Pharmacol. 674, 294–306 (2012).
Porter, D. W., Irwin, N., Flatt, P. R., Holscher, C. & Gault, V. A. Prolonged GIP receptor activation improves cognitive function, hippocampal synaptic plasticity and glucose homeostasis in high-fat fed mice. Eur. J. Pharmacol. 650, 688–693 (2011).
Li, Y., Liu, W., Li, L. & Holscher, C. D-Ala2-GIP-glu-PAL is neuroprotective in a chronic Parkinson’s disease mouse model and increases BNDF expression while reducing neuroinflammation and lipid peroxidation. Eur. J. Pharmacol. 797, 162–172 (2017).
Verma, M. K., Goel, R., Nandakumar, K. & Nemmani, K. V. S. Bilateral quinolinic acid-induced lipid peroxidation, decreased striatal monoamine levels and neurobehavioral deficits are ameliorated by GIP receptor agonist D-Ala(2)GIP in rat model of Huntington’s disease. Eur. J. Pharmacol. 828, 31–41 (2018).
Yuan, L. et al. DAla2-GIP-GLU-PAL protects against cognitive deficits and pathology in APP/PS1 mice by inhibiting neuroinflammation and upregulating cAMP/PKA/CREB signaling pathways. J. Alzheimers Dis. 80, 695–713 (2021).
Yang, X. et al. Neuroprotective mechanisms of glucagon-like peptide-1-based therapies in ischemic stroke: an update based on preclinical research. Front. Neurol. 13, 844697 (2022).
Aviles-Olmos, I. et al. Exenatide and the treatment of patients with Parkinson’s disease. J. Clin. Invest. 123, 2730–2736 (2013).
Athauda, D. et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 390, 1664–1675 (2017).
Vijiaratnam, N. et al. Exenatide once weekly over 2 years as a potential disease-modifying treatment for Parkinson’s disease: protocol for a multicentre, randomised, double blind, parallel group, placebo controlled, phase 3 trial: the ‘Exenatide-PD3’ study. BMJ Open 11, e047993 (2021).
Watson, K. T. et al. Neural correlates of liraglutide effects in persons at risk for Alzheimer’s disease. Behav. Brain Res. 356, 271–278 (2019).
Cukierman-Yaffe, T. et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 19, 582–590 (2020).
Norgaard, C. H. et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement. 8, e12268 (2022).
Gasbjerg, L. S. et al. Separate and combined glucometabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes 68, 906–917 (2019).
Frias, J. P. et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med. 385, 503–515 (2021).
Jastreboff, A. M. et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 387, 205–216 (2022).
Gastaldelli, A. et al. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS-3 MRI): a substudy of the randomised, open-label, parallel-group, phase 3 SURPASS-3 trial. Lancet Diabetes Endocrinol. 10, 393–406 (2022).
Finan, B. et al. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med. 5, 209ra151 (2013).
Mroz, P. A. et al. Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Mol. Metab. 20, 51–62 (2019).
Bergmann, N. C. et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: a randomised, crossover study. Diabetologia 62, 665–675 (2019).
Yuan, Z. et al. A novel GLP-1/GIP dual agonist is more effective than liraglutide in reducing inflammation and enhancing GDNF release in the MPTP mouse model of Parkinson’s disease. Eur. J. Pharmacol. 812, 82–90 (2017).
Li, T. et al. A GLP-1/GIP/Gcg receptor triagonist improves memory behavior, as well as synaptic transmission, neuronal excitability and Ca(2+) homeostasis in 3xTg-AD mice. Neuropharmacology 170, 108042 (2020).
Borner, T. et al. GIP receptor agonism attenuates GLP-1 receptor agonist induced nausea and emesis in preclinical models. Diabetes 70, 2545–2553 (2021).
Zhang, C., Vincelette, L. K., Reimann, F. & Liberles, S. D. A brainstem circuit for nausea suppression. Cell Rep. 39, 110953 (2022).
Wilson, J. M. et al. The dual glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist tirzepatide improves cardiovascular risk biomarkers in patients with type 2 diabetes: a post hoc analysis. Diabetes Obes. Metab. 24, 148–153 (2022).
Sattar, N. et al. Tirzepatide cardiovascular event risk assessment: a pre-specified meta-analysis. Nat. Med. 28, 591–598 (2022).
Miyawaki, K. et al. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc. Natl Acad. Sci. USA 96, 14843–14847 (1999).
Lu, S. C. et al. GIPR antagonist antibodies conjugated to GLP-1 peptide are bispecific molecules that decrease weight in obese mice and monkeys. Cell Rep. Med. 2, 100263 (2021).
N’Diaye, N., Tremblay, J., Hamet, P., De Herder, W. W. & Lacroix, A. Adrenocortical overexpression of gastric inhibitory polypeptide receptor underlies food-dependent Cushing’s syndrome. J. Clin. Endocrinol. Metab. 83, 2781–2785 (1998).
Regazzo, D. et al. The GIP/GIPR axis is functionally linked to GH-secretion increase in a significant proportion of gsp(−) somatotropinomas. Eur. J. Endocrinol. 176, 543–553 (2017).
Harada, N. et al. A novel GIP receptor splice variant influences GIP sensitivity of pancreatic beta-cells in obese mice. Am. J. Physiol. Endocrinol. Metab. 294, E61–E68 (2008).
Ahlqvist, E. et al. Link between GIP and osteopontin in adipose tissue and insulin resistance. Diabetes 62, 2088–2094 (2013).
DeBoever, C. et al. Medical relevance of common protein-altering variants in GPCR genes across 337,205 individuals in the UK Biobank. Preprint at bioRxiv https://doi.org/10.1101/2019.12.13.876250 (2020).
Harris, M. et al. RAMPs regulate signalling bias and internalisation of the GIPR. Preprint at bioRxiv https://doi.org/10.1101/2021.04.08.436756 (2021).
Lagou, V. et al. Random glucose GWAS in 493,036 individuals provides insights into diabetes pathophysiology, complications and treatment stratification. Preprint at medRxiv https://doi.org/10.1101/2021.04.17.21255471 (2021).
Dawed, A. Y. et al. Pharmacogenomics of GLP-1 receptor agonists: a genome wide analysis of observational data and large randomized controlled trials. Preprint at medRxiv https://doi.org/10.1101/2022.05.27.22271124 (2022).
Acknowledgements
D.J.D. acknowledges the support of the Banting and Best Diabetes Centre Novo Nordisk Chair in Incretin Biology, the Sinai Health-Novo Nordisk Foundation Fund in regulatory peptides and CIHR grant number 154321. R.H. acknowledges the support of a fellowship from the Banting and Best Diabetes Centre.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
D.J.D. has received speaking or consulting funds from Altimmune, Amgen, Eli Lilly, Kallyope, Merck, Novo Nordisk Inc. and Pfizer Inc. Preclinical studies in the Drucker laboratory are funded in part by investigator-initiated operating grants to Sinai Health from Novo Nordisk Inc. and Pfizer Inc. R.H. declares no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Juan Pablo Frias, Baptist Gallwitz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hammoud, R., Drucker, D.J. Beyond the pancreas: contrasting cardiometabolic actions of GIP and GLP1. Nat Rev Endocrinol 19, 201–216 (2023). https://doi.org/10.1038/s41574-022-00783-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-022-00783-3
This article is cited by
-
Effects of finerenone and glucagon-like peptide 1 receptor agonists on cardiovascular and renal outcomes in type 2 diabetes mellitus: a systematic review and meta-analysis
Diabetology & Metabolic Syndrome (2024)
-
Combination therapy for kidney disease in people with diabetes mellitus
Nature Reviews Nephrology (2024)
-
GIP regulates body weight via GABAergic neurons
Nature Reviews Endocrinology (2024)
-
Inter-organ crosstalk during development and progression of type 2 diabetes mellitus
Nature Reviews Endocrinology (2024)
-
Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases
Signal Transduction and Targeted Therapy (2024)