Abstract
Objectives:
Metabolically obese but normal weight (MONW) individuals constitute a subgroup of normal weight individuals that display impaired insulin sensitivity with a higher risk of developing diabetes, cardiovascular disease and mortality. We aimed to propose a novel criterion for defining MONW by examining the usefulness and the cutoff value of the TyG index, a product of the levels of triglycerides and glucose, in identifying MONW individuals. In addition, the performance of this criterion in predicting the future incidence of diabetes was assessed.
Subjects/methods:
A total of 7541 non-diabetic, normal weight (body mass index ⩾18.5 and <25 kg m−2) subjects were selected from the Korea National Health and Nutrition Examination Survey conducted in 2009–2010. Another 3185 participants with follow-up studies were selected from a prospective community-based cohort study. The TyG index was calculated as ln(fasting triglycerides (mg dl−1) × fasting glucose (mg dl−1)/2).
Results:
The levels of the TyG index paralleled the prevalence of metabolic syndrome and its components. The cutoff value of the TyG index that reflected MONW based on the receiver operating characteristics analysis was 8.82 for men and 8.73 for women, with the area under the curve values being 0.855 and 0.868, respectively. The sensitivity and the specificity were 84.2 and 77.6% in men and 69.1 and 89.4% in women, respectively. Individuals designated as MONW, who have a normal weight and TyG levels higher than cutoff, displayed a metabolically unhealthy phenotype and an approximately twofold higher risk of developing diabetes compared with metabolically healthy normal weight subjects.
Conclusions:
We propose a simple diagnostic criterion of MONW, which might be used to discriminate subjects with a higher risk of metabolic diseases.
Similar content being viewed by others
Introduction
The rapid increase in the prevalence of obesity is leading to an epidemic of various metabolic diseases, causing enormous health and socioeconomic burden worldwide.1, 2, 3 Although the body mass index (BMI) has been widely used as a reliable and simple measure of defining obesity, recent studies identified that some subpopulations show unexpected metabolic profiles that deviate from the typical dose-response relationship between BMI and metabolic disturbances.4, 5, 6, 7, 8, 9, 10
The concept that some non-obese or slightly obese individuals present with several risk factors for metabolic disorders and represent one end of the spectrum of obesity was first proposed by Ruderman et al.4 ~30 years ago. Ongoing investigations reveal that these metabolically obese but normal weight (MONW) individuals, also called metabolically abnormal normal weight or normal weight obesity, are not uncommon.9, 11 They are characterized by the increased levels of adiposity and insulin resistance and a higher susceptibility to type 2 diabetes and cardiovascular diseases (CVD).9 Furthermore, elderly people with the MONW phenotype exhibited a higher risk of all-cause and CVD mortality.12 In this regard, early identification of MONW individuals would have significant benefits by guiding appropriate risk management. However, a consensus on the definition of MONW is lacking, and measuring the degree of insulin resistance or adiposity, which is the core characteristic of MONW, is not widely applicable. We previously showed that MONW individuals could be identified based on lipid profiles11 or the TyG index, the product of the fasting blood glucose and triglyceride (TG) levels.13 The TyG index is a simple marker that correlates well with the degree of insulin resistance measured by hyperinsulinemic-euglycemic clamp studies.14, 15 Because a higher degree of insulin resistance is a representative feature of MONW, using the TyG index might help identifying these individuals.
In this study, we aimed to clarify the usefulness and the cutoff value of the TyG index in identifying MONW individuals using the nationwide survey data from Korea. Based on our analyses, we propose a simple diagnostic criterion for MONW. In addition, the performance of this novel criterion in predicting the future incidence of diabetes was assessed in another prospective cohort study.
Subjects and methods
Subjects
Data acquired from two cohort studies were used. The first cohort was a cross-sectional study from the Korea National Health and Nutrition Examination Survey (KNHANES) IV and V, which was conducted in 2009–2010. The Korean Ministry of Health and Welfare and the Korea Centers for Disease Control have conducted the KNHANES periodically since 1998 and annually since 2008. It consists of a health interview survey, a health examination survey and a nutrition survey, which are administered by trained investigators. A rolling sampling design that involves complex, stratified, multistage probability samples is used to collect information that represents the non-institutionalized civilian population in South Korea. Additional details regarding the survey are provided elsewhere.16, 17 Among the 14 633 subjects aged 19 years or older who participated in the survey, only non-diabetic, normal weight (BMI ⩾18.5 and <25 kg m−2) subjects were selected for the analysis. Participants with malignancy, chronic liver disease, renal insufficiency, those who are pregnant and those lacking data for variables included in the analysis were excluded. After excluding the ineligible subjects, the total number of participants for this analysis was 7541 (2987 men and 4554 women). Written informed consent was obtained from all participants, and the institutional review board at The Catholic University of Korea approved this study (No. KC13SISI0796).
To test whether the new criterion for MONW is useful for predicting the development of diabetes, we used the data obtained from the Chungju Metabolic disease Cohort (CMC) study (cohort 2). This study is an ongoing community-based study started in 2003 in a population aged 40 years and over living in the rural area of Chungju City, Korea. Among the 5354 subjects who showed normal glucose tolerance at baseline and had completed their follow-up visit at least once until March 2013 with a median (minimum, maximum) duration of follow-up of 4.6 (4.0, 8.8) years, only normal weight individuals (n=3185) were selected for the analysis. Additional details regarding this cohort study are described elsewhere.11, 18
Measurements
Anthropometric measurements were performed while the participants wore light clothing, and the body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively. The BMI was calculated as the weight (kg) divided by the height (m) squared. The waist circumference (WC) was measured to the nearest 0.1 cm at the level of the midpoint between the iliac crest and the costal margin at the end of a normal expiration. The blood pressure (BP) was measured three times on the right arm with a mercury sphygmomanometer (Baumanometer; Baum, Copiague, NY, USA) while the subjects were in a seated position after at least 5 min of rest. The final BP value was obtained by averaging the values of the second and third measurements. Hypertension was defined by using the Joint National Committee 7 report as ⩾140 (systolic BP)/90 (diastolic BP) mmHg or when the subjects reported using antihypertensive medications. Smoking was defined as current smokers who had used at least 5 packs of cigarettes ever. Subjects who drank 15~30 g per day of alcohol were designated as mild-to-moderate drinkers, and those who drank more than 30 g per day were classified as heavy drinkers. Regular exercise was defined as a strenuous physical activity performed for at least 20 min at a time, at least three times per week. The percentage of body fat and appendicular skeletal muscle mass were measured using dual-energy X-ray absorptiometry (QDR 4500 A, Hologic Inc., Waltham, MA, USA) located in mobile examination centers. Blood samples were obtained after a minimum fasting time of 8 h. The serum insulin level was measured by an immunoradiometric assay (INS-IRMA; Biosource, Nivelles, Belgium). The serum levels of glucose, total cholesterol, TG and high-density lipoprotein cholesterol were measured enzymatically using a Hitachi automatic analyzer 7600 (Tokyo, Japan), and the low-density lipoprotein cholesterol was calculated using the Friedewald formula. The coefficient of variation of the assays was <5%. The homeostasis model assessment estimate of insulin resistance was calculated using the following formula: fasting blood glucose (mmol l−1) × fasting insulin (mIU l−1)/22.5. The TyG index was calculated as ln(fasting triglycerides (mg dl−1) × fasting glucose (mg dl−1)/2).14, 15
Definition of metabolic syndrome, impaired fasting glucose and diabetes mellitus
Metabolic syndrome was defined in accordance with the American Heart Association/National Heart, Lung and Blood Institute criteria,19 including a modification in the WC value according to the World Health Organization–Asian Pacific region criteria for abdominal obesity. This entity was defined if three or more of the criteria were present, as follows: (1) WC ⩾90 cm for men and ⩾80 cm for women; (2) fasting TG ⩾1.69 mmol l−1 or antihyperlipidemic medication use; (3) high-density lipoprotein-cholesterol <1.03 mmol l−1 for men and <1.29 mmol l−1 for women or antihyperlipidemic medication use; (4) BP ⩾130/85 mm Hg or antihypertensive medication use; and (5) fasting plasma glucose ⩾5.6 mmol l−1. Subjects having a fasting blood glucose level between 100 and 125 mg dl−1 were classified as impaired fasting glucose. In cohort 2, the diagnosis of incident diabetes mellitus was made based on the American Diabetes Association criteria. An oral glucose tolerance test was performed if the fasting plasma glucose level exceeded 5.6 mmol l−1. However, every participant who had followed-up after September 2009 underwent an oral glucose tolerance test, independent of their fasting plasma glucose levels to minimize the possibility of underestimating the incidence of diabetes mellitus.
Definition of MONW
Three different definitions of being ‘metabolically obese’ were used as appropriate in this study. Individuals within a normal BMI (⩾18.5 and <25 kg m−2) range (1) having metabolic syndrome (metabolic syndrome criterion), (2) with a TyG index higher than the cutoff value (TyG criterion) or (3) with homeostasis model assessment estimate of insulin resistance in the highest quartile (homeostasis model assessment criterion) were designated as MONW. Subjects not fulfilling these criteria were allocated in the metabolically healthy and normal weight (MHNW) group.
Statistical analysis
All statistical analyses were performed using the SAS 9.3 software (SAS Institute Inc., Cary, NC, USA). The SAS survey procedure was used to account for the complex sampling design and sampling weights of KNHANES and to provide nationally representative prevalence estimates. For the subgroup analysis, a domain analysis was applied to preserve the complex sampling design, in which entire samples were used to estimate the variance of the subpopulations. The data are expressed as the means±s.e., as geometric means (95% confidence interval (CI)) or as proportions. If necessary, a logarithmic transformation was performed to achieve a normal distribution. To compare the characteristics of subgroups, an independent sample Student’s t-test or χ2-test was used. The area under the curve and a 95% CI of the receiver operating characteristics curve were calculated to define the cutoff values of the TyG index in identifying MONW individuals. The optimal cutoff values were determined from the maximal Youden’s Index (sensitivity+specificity−1). Multiple logistic regression analysis was used to determine the odds ratios and 95% CI values of cardiovascular risk factors in the MONW subjects, with the MHNW group as the reference category. A log-binomial regression model using Proc GenMod procedure was performed to calculate the relative risk (RR) of MONW in predicting the development of diabetes, with MHNW as the reference category. A P-value <0.05 was considered significant.
Results
The baseline characteristics of the study subjects are described in Table 1. The mean age and BMI were 42.5±0.4 years and 22.15±0.04 kg m−2 for men and 42.5±0.4 years and 21.53±0.04 kg m−2 for women, respectively. The mean blood glucose levels (5.11±0.01 vs 4.98±0.01 mmol l−1, P<0.0001), geometric mean of TG levels (1.24 (1.20–1.27) vs 0.92 (0.90–0.93) mmol l−1, P<0.0001) and the TyG index (8.53±0.02 vs 8.21±0.01, P<0.0001) were significantly higher in men than women. Impaired fasting glucose and hypertension, but not metabolic syndrome, were more prevalent in men than women. Because significant differences were noted in most of the anthropometric measures, body composition, blood parameters and lifestyle factors between men and women, all further analyses were performed separately.
Next, the prevalence of metabolic syndrome and its components according to the TyG index deciles were analyzed (Figure 1). The percentage of subjects with metabolic syndrome steeply increased from the eighth decile in men and nineth decile in women. This increase paralleled an abrupt increase in the percentage of high TG levels. In contrast, the prevalence of other components of metabolic syndrome gradually increased according to the TyG index deciles. A high BP and low high-density lipoprotein cholesterol level were the most prevalent component in men and women, respectively.
The prevalence of metabolic syndrome and its components according to the TyG index deciles in men (a) and women (b). The numbers on the x axis represents the cutoff values of each decile. BP, blood pressure; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; MetS, metabolic syndrome; TG, triglyceride; WC, waist circumference.
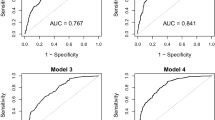
To determine the cutoff value of the TyG index in identifying MONW individuals, a receiver operating characteristics analysis was performed using MONW defined by the metabolic syndrome criterion as a reference (Figure 2). The optimal cutoff value was 8.82 for men and 8.73 for women, with area under the curve (95% CI) values of 0.855 (0.842–0.868) and 0.868 (0.857–0.877), respectively. The sensitivity, specificity, positive predictive value and negative predictive value were 84.2, 77.6, 34.0 and 97.3% in men and 69.1, 89.4, 49.0 and 95.2% in women, respectively. The area under the curve value of the TyG index was significantly higher than that of TG (0.828) or glucose (0.808) in men and that of glucose (0.753) in women.
The gender-specific cutoff values of the TyG index were used to classify participants as MONW or MHNW. The degree of obesity measured by BMI, WC, waist–height ratio and the percentage of total body fat were significantly higher in MONW individuals than in MHNW individuals (Table 2). MONW individuals were more sarcopenic, as revealed by lower levels of the appendicular skeletal muscle and appendicular skeletal muscle/weight ratio. Subjects in the MONW group were more hypertensive, hyperglycemic, hyperlipidemic and insulin resistant. These findings were largely identical in both men and women. The prevalence of CHD and stroke was higher in the MONW group than in the MHNW group, but this difference was only significant in women. Furthermore, the odds of having impaired fasting glucose, hypertension and metabolic syndrome were significantly higher in the MONW group, even after adjusting for age, BMI, regular exercise, alcohol drinking and smoking status (Table 3). Our data show that the clinical characteristics of the MONW group defined using the TyG criterion generally follow the known phenotypes of MONW defined by other criteria. This finding suggests that the TyG index clearly discriminates individuals at metabolic risk among the normal weight population.
Next, the performance of this new criterion for MONW in predicting the incidence of diabetes was tested in another community-based prospective cohort study. In both men and women, the incidence of diabetes was significantly higher in the MONW group compared with the MHNW group (men, 11.95% (52/435) vs 5.79% (55/950), P<0.0001; women, 9.29% (65/700) vs 3.45% (38/1100), P<0.0001). Among the non-diabetic normal weight population, subjects in the MONW group were at a significantly higher risk of developing diabetes (Table 4). In the crude analysis, the risk of diabetes was more than twofold higher in the MONW group than in the MHNW group in both men (RR, 2.21; 95% CI, 1.49–3.29) and women (RR, 2.86; 95% CI, 1.90–4.32). This association was maintained even after adjustment for age, BMI, systolic BP, high-density lipoprotein cholesterol, WC, family history of diabetes, alcohol drinking and smoking status. The homeostasis model assessment criterion of MONW was also useful in predicting the incidence of diabetes, with a RR of ~1.6. However, the TyG criterion was more predictive than the homeostasis model assessment criterion, with a higher RR in both men and women.
Discussion
In the current study, we tested the role of the TyG index in identifying MONW subjects. By determining the cutoff value of the TyG index in this context, we propose that individuals with a higher TyG index (above 8.82 for men and 8.73 for women) and normal weight (BMI ⩾18.5 and <25 kg m−2) can be classified into the MONW group. This novel criterion successfully reflected the metabolic phenotype of MONW and predicted the future development of diabetes.
Recently, subgroups of obesity, such as MONW or metabolically healthy obesity, have gained much interest as their long-term consequences are beginning to be elucidated. Although the notion that metabolically healthy obesity phenotype individuals are protected from diabetes, CVD or mortality compared with metabolically unhealthy obese people is controversial,9, 20, 21, 22, 23 subjects with the MONW phenotype are definitely regarded as a high-risk group among the normal weight population. They are susceptible to developing diabetes and CVD,9, 20, 24 which leads to increased CVD- or all-cause mortality.12, 23, 25, 26 Furthermore their clinical characteristics are being studied in detail. In addition to the well-known phenotypes of MONW, such as impaired insulin sensitivity, increased visceral adiposity, higher levels of BP, atherogenic lipid profiles and a lower physical activity energy expenditure,5, 6, 7, 11, 26, 27 they also show a higher degree of subclinical vascular inflammation, an increased arterial stiffness and carotid atherosclerosis, higher levels of oxidative stress, an unfavorable adipokine profile and impaired left ventricular function.28, 29, 30, 31, 32 Considering these observations, the early identification of individuals with the MONW phenotype would be beneficial and may have important therapeutic implications.
However, unified criteria that define this distinct subset are lacking, resulting in a wide variation in the prevalence of MONW.11 The definition that best predicts adverse outcomes is also currently unclear. In previous studies, the ‘metabolically obese’ status has been defined as having metabolic syndrome,9, 33, 34 being in the highest quartile or in the specific range of homeostasis model assessment estimate of insulin resistance,6, 9, 11 having excess visceral or body fat,35, 36 showing a decreased glucose disposal rate,37, 38 or having multiple cardiovascular risk factors.39 These definitions and the clinical characteristics suggest that the main component of MONW is insulin resistance. In this regard, we examined the possibility of using the TyG index, which has been shown to correlate well with other measures of insulin sensitivity,14, 15 as a simple criterion in defining MONW. Our previous analyses showed that the odds ratios of being categorized into the MONW group increased in a stepwise manner across the TyG index quartiles among normal weight subjects.13 In this study, we obtained the optimal cutoff values of the TyG index in identifying MONW using the metabolic syndrome criterion as a reference. The receiver operating characteristics analysis showed high area under the curve of receiver operating characteristics values, sensitivity and specificity of the determined TyG cutoff, suggesting that this index is highly predictive. When the participants were divided into MHNW and MONW using the TyG criterion, their clinical characteristics were revealed to clearly differ. A higher metabolic risks and prevalence of impaired fasting glucose, hypertension and metabolic syndrome were noted. Applying the new criterion in a prospective cohort study demonstrated that MONW men and women defined by the TyG criterion were at a significantly higher risk of developing diabetes than MHNW men and women. The TyG criterion outperformed the homeostasis model assessment criterion with a higher RR. Therefore, the TyG criterion might be useful to discriminate individuals with a higher risk of metabolic diseases among apparently healthy-looking normal weight subjects. The most advantageous point of this criterion is that it is an easily calculated measure without the need for complex studies, measuring the BP, WC or serum insulin levels. Hence, it is widely applicable in large epidemiologic studies or in public health management.
The strength of this study is that nationwide survey data involving large number of subjects representing Koreans were analyzed. We further confirmed the performance of the TyG criterion in a prospective community-based cohort study. However, our study features some limitations. First, we did not explore whether MONW individuals defined using the TyG criterion also have higher risk of CVD or mortality. Long-term outcome studies will be needed to elucidate this issue. Second, the CMC study used to assess the prediction of the incidence of diabetes by the TyG criterion consisted of middle aged or elderly participants over 40 years old, which limited the interpretation in younger people. Third, because all individuals in this study were Koreans, the applicability and utility of the TyG criterion in other ethnic populations need to be further confirmed. The definitions of overweight and obese status, as well as the TG levels differ across different ethnicities.40 Furthermore, the susceptibility to metabolic diseases is thought to be higher in Asians for similar degrees of obesity.
In conclusion, our study provides evidence for using the TyG index to identify a metabolically risky subgroup among the normal weight population. Thereby, we propose a novel criterion for defining MONW, which could be widely utilized due to its simplicity, and show its effectiveness in predicting the incidence of diabetes. Outcome studies to evaluate the predictability of this definition on CVD and mortality and confirmation in other cohort studies are warranted. Individualized management via the proper stratification of normal weight individuals according to their metabolic status will be of great importance in the future.
References
Pi-Sunyer FX . The obesity epidemic: pathophysiology and consequences of obesity. Obes Res 2002; 10 (Suppl 2): 97s–104s.
Kim CS, Ko SH, Kwon HS, Kim NH, Kim JH, Lim S et al. Prevalence, awareness, and management of obesity in Korea: data from the Korea national health and nutrition examination survey (1998-2011). Diabetes Metab J 2014; 38: 35–43.
Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M . Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 2011; 378: 815–825.
Ruderman NB, Schneider SH, Berchtold P . The ‘metabolically-obese,’ normal-weight individual. Am J Clin Nutr 1981; 34: 1617–1621.
Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S . The metabolically obese, normal-weight individual revisited. Diabetes 1998; 47: 699–713.
Conus F, Allison DB, Rabasa-Lhoret R, St-Onge M, St-Pierre DH, Tremblay-Lebeau A et al. Metabolic and behavioral characteristics of metabolically obese but normal-weight women. J Clin Endocrinol Metab 2004; 89: 5013–5020.
Karelis AD, St-Pierre DH, Conus F, Rabasa-Lhoret R, Poehlman ET . Metabolic and body composition factors in subgroups of obesity: what do we know? J Clin Endocrinol Metab 2004; 89: 2569–2575.
Karelis AD, Faraj M, Bastard JP, St-Pierre DH, Brochu M, Prud'homme D et al. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab 2005; 90: 4145–4150.
Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 2006; 91: 2906–2912.
Kuk JL, Ardern CI . Are metabolically normal but obese individuals at lower risk for all-cause mortality? Diabetes Care 2009; 32: 2297–2299.
Lee SH, Ha HS, Park YJ, Lee JH, Yim HW, Yoon KH et al. Identifying metabolically obese but normal-weight (MONW) individuals in a nondiabetic Korean population: the Chungju Metabolic disease Cohort (CMC) study. Clin Endocrinol (Oxf) 2011; 75: 475–481.
Choi KM, Cho HJ, Choi HY, Yang SJ, Yoo HJ, Seo JA et al. Higher mortality in metabolically obese normal-weight people than in metabolically healthy obese subjects in elderly Koreans. Clin Endocrinol (Oxf) 2013; 79: 364–370.
Lee SH, Han K, Yang HK, Kim MK, Yoon KH, Kwon HS et al. Identifying subgroups of obesity using the product of triglycerides and glucose: the Korea National Health and Nutrition Examination Survey, 2008-2010. Clin Endocrinol (Oxf) 2015; 82: 213–220.
Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab 2010; 95: 3347–3351.
Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F . The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 2008; 6: 299–304.
Kang JH, Kim SS, Moon SS, Kim WJ, Bae MJ, Choi BG et al. Adiposity in the relationship between serum vitamin D level and insulin resistance in middle-aged and elderly Korean adults: the Korea National Health and Nutrition Examination Survey 2008. Endocrinol Metab (Seoul) 2013; 28: 96–102.
Lee J, Lee S, Jang S, Ryu OH . Age-related changes in the prevalence of osteoporosis according to gender and skeletal site: the Korea National Health and Nutrition Examination Survey 2008-2010. Endocrinol Metab (Seoul) 2013; 28: 180–191.
Lee SH, Kwon HS, Park YM, Ha HS, Jeong SH, Yang HK et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS One 2014; 9: e90430.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735–2752.
Aung K, Lorenzo C, Hinojosa MA, Haffner SM . Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab 2014; 99: 462–468.
Durward CM, Hartman TJ, Nickols-Richardson SM . All-cause mortality risk of metabolically healthy obese individuals in NHANES III. J Obes 2012; 2012: 460321.
Hamer M, Stamatakis E . Metabolically healthy obesity and risk of all-cause and cardiovascular disease mortality. J Clin Endocrinol Metab 2012; 97: 2482–2488.
Hinnouho GM, Czernichow S, Dugravot A, Batty GD, Kivimaki M, Singh-Manoux A . Metabolically healthy obesity and risk of mortality: does the definition of metabolic health matter? Diabetes Care 2013; 36: 2294–2300.
Rhee EJ, Lee MK, Kim JD, Jeon WS, Bae JC, Park SE et al. Metabolic health is a more important determinant for diabetes development than simple obesity: a 4-year retrospective longitudinal study. PLoS One 2014; 9: e98369.
Batsis JA, Sahakyan KR, Rodriguez-Escudero JP, Bartels SJ, Somers VK, Lopez-Jimenez F . Normal weight obesity and mortality in United States subjects ⩾60 years of age (from the Third National Health and Nutrition Examination Survey). Am J Cardiol 2013; 112: 1592–1598.
Romero-Corral A, Somers VK, Sierra-Johnson J, Korenfeld Y, Boarin S, Korinek J et al. Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 2010; 31: 737–746.
Conus F, Rabasa-Lhoret R, Péronnet F . Characteristics of metabolically obese normal-weight (MONW) subjects. Appl Physiol Nutr Metab 2007; 32: 4–12.
Kang S, Kyung C, Park JS, Kim S, Lee SP, Kim MK et al. Subclinical vascular inflammation in subjects with normal weight obesity and its association with body fat: an 18 F-FDG-PET/CT study. Cardiovasc Diabetol 2014; 13: 70.
Kosmala W, Jedrzejuk D, Derzhko R, Przewlocka-Kosmala M, Mysiak A, Bednarek-Tupikowska G . Left ventricular function impairment in patients with normal-weight obesity: contribution of abdominal fat deposition, profibrotic state, reduced insulin sensitivity, and proinflammatory activation. Circ Cardiovasc Imaging 2012; 5: 349–356.
Yoo HJ, Hwang SY, Hong HC, Choi HY, Seo JA, Kim SG et al. Association of metabolically abnormal but normal weight (MANW) and metabolically healthy but obese (MHO) individuals with arterial stiffness and carotid atherosclerosis. Atherosclerosis 2014; 234: 218–223.
Hyun YJ, Koh SJ, Chae JS, Kim JY, Kim OY, Lim HH et al. Atherogenecity of LDL and unfavorable adipokine profile in metabolically obese, normal-weight woman. Obesity (Silver Spring) 2008; 16: 784–789.
Katsuki A, Sumida Y, Urakawa H, Gabazza EC, Murashima S, Nakatani K et al. Increased oxidative stress is associated with serum levels of triglyceride, insulin resistance, and hyperinsulinemia in Japanese metabolically obese, normal-weight men. Diabetes Care 2004; 27: 631–632.
Lee K . Metabolically obese but normal weight (MONW) and metabolically healthy but obese (MHO) phenotypes in Koreans: characteristics and health behaviors. Asia Pac J Clin Nutr 2009; 18: 280–284.
St-Onge MP, Janssen I, Heymsfield SB . Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care 2004; 27: 2222–2228.
Katsuki A, Sumida Y, Urakawa H, Gabazza EC, Murashima S, Maruyama N et al. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care 2003; 26: 2341–2344.
Kim MK, Han K, Kwon HS, Song KH, Yim HW, Lee WC et al. Normal weight obesity in Korean adults. Clin Endocrinol (Oxf) 2014; 80: 214–220.
Dvorak RV, DeNino WF, Ades PA, Poehlman ET . Phenotypic characteristics associated with insulin resistance in metabolically obese but normal-weight young women. Diabetes 1999; 48: 2210–2214.
McLaughlin T, Allison G, Abbasi F, Lamendola C, Reaven G . Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism 2004; 53: 495–499.
Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999-2004). Arch Intern Med 2008; 168: 1617–1624.
Lin SX, Carnethon M, Szklo M, Bertoni A . Racial/ethnic differences in the association of triglycerides with other metabolic syndrome components: the Multi-Ethnic Study of Atherosclerosis. Metab Syndr Relat Disord 2011; 9: 35–40.
Acknowledgements
We wish to thank all the team members and survey personnel of the CMC study and the officers in Chungju Health Center for continued support. This study was supported by the grants from Handok, Inc., The Catholic Medical Center Research Foundation made in the program year of 2014 (to S-HL) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP, 2013M3C8A2075675; to K-HY).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Lee, SH., Han, K., Yang, H. et al. A novel criterion for identifying metabolically obese but normal weight individuals using the product of triglycerides and glucose. Nutr & Diabetes 5, e149 (2015). https://doi.org/10.1038/nutd.2014.46
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nutd.2014.46
This article is cited by
-
Comparison of the prognostic value of a comprehensive set of predictors in identifying risk of metabolic-associated fatty liver disease among employed adults
BMC Public Health (2023)
-
Obesity phenotypes and cardiovascular risk: From pathophysiology to clinical management
Reviews in Endocrine and Metabolic Disorders (2023)
-
Association of triglyceride glucose index with all-cause and cause-specific mortality among middle age and elderly US population
BMC Geriatrics (2022)
-
RETRACTED ARTICLE: Association of metabolic syndrome with TyG index and TyG-related parameters in an urban Chinese population: a 15-year prospective study
Diabetology & Metabolic Syndrome (2022)
-
Association of triglyceride-glucose index with clinical outcomes in patients with acute ischemic stroke receiving intravenous thrombolysis
Scientific Reports (2022)