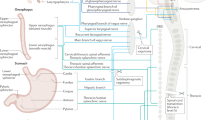
Abstract
The primary function of the gut is to procure nutrients. Synchronized mechanical activities underlie nearly all its endeavours. Coordination of mechanical activities depends on sensing of the mechanical forces, in a process called mechanosensation. The gut has a range of mechanosensory cells. They function either as specialized mechanoreceptors, which convert mechanical stimuli into coordinated physiological responses at the organ level, or as non-specialized mechanosensory cells that adjust their function based on the mechanical state of their environment. All major cell types in the gastrointestinal tract contain subpopulations that act as specialized mechanoreceptors: epithelia, smooth muscle, neurons, immune cells, and others. These cells are tuned to the physical properties of the surrounding tissue, so they can discriminate mechanical stimuli from the baseline mechanical state. The importance of gastrointestinal mechanosensation has long been recognized, but the latest discoveries of molecular identities of mechanosensors and technical advances that resolve the relevant circuitry have poised the field to make important intellectual leaps. This Review describes the mechanical factors relevant for normal function, as well as the molecules, cells and circuits involved in gastrointestinal mechanosensing. It concludes by outlining important unanswered questions in gastrointestinal mechanosensing.
Key points
-
Mechanosensation is the ability to sense mechanical forces and transduce them into physiological responses.
-
The gut is a mechanically active organ in which all cells must sense the forces emanating from the digestion of intraluminal contents and organ activity, such as motility.
-
All cells reside in tissue at a baseline mechanical state; the gastrointestinal tract is a layered (composite) organ in which the baseline mechanical state varies by spatial localization.
-
Non-specialized mechanosensory cells sense force to adjust their function; specialized mechanoreceptors are mechanosensory cells that guide physiological organ responses to mechanical stimuli.
-
Gastrointestinal mechanoreceptors share similarities with mechanoreceptors in other sensory and non-sensory organs; leveraging these similarities helps in understanding the purpose and function of mechanoreceptors in the gastrointestinal tract.
-
Mechanosensory circuits built into the gastrointestinal wall allow for spatial and temporal integration of mechanical stimuli into a coordinated physiological response (for example, peristaltic reflex), and connections to the extrinsic mechanosensory circuits are crucial for brain–gut communication (for example, sense of fullness).
-
Areas with research potential include the discovery of unknown mechanosensors, quantification of the baseline mechanical state of the gastrointestinal wall, and the development of novel tests for gut-specific mechanosensation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cannon, W. B. The Mechanical Factors of Digestion (Longmans, Green & Co., 1911).
Alvarez, W. C. The Mechanics of the Digestive Tract: An Introduction to Gastroenterology. 2nd edn (Hoeber, 1928).
Bayliss, W. M. & Starling, E. H. The movements and innervation of the small intestine. J. Physiol. 24, 99–143 (1899).
Ingber, D. E. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 20, 811–827 (2006).
Gregersen, H. Biomechanics of the Gastrointestinal Tract: New Perspectives in Motility Research and Diagnostics (Springer, 2003).
Stevens, C. E. & Hume, I. D. Comparative Physiology of the Vertebrate Digestive System. 2nd edn (Cambridge Univ. Press, 2004).
Liao, D., Zhao, J. & Gregersen, H. Three-dimensional geometry analysis of the stomach in type II diabetic GK rats. Diabetes Res. Clin. Pract. 71, 1–13 (2006).
Chen, X., Zhao, J. & Gregersen, H. The villi contribute to the mechanics in the guinea pig small intestine. J. Biomech. 41, 806–812 (2008).
Zhao, J., Nakaguchi, T. & Gregersen, H. Biomechanical and histomorphometric colon remodelling in STZ-induced diabetic rats. Dig. Dis. Sci. 54, 1636–1642 (2009).
Dinning, P. G., Arkwright, J. W., Gregersen, H., O’Grady, G. & Scott, S. M. Technical advances in monitoring human motility patterns. Neurogastroenterol. Motil. 22, 366–380 (2010).
Gao, C. & Gregersen, H. Biomechanical and morphological properties in rat large intestine. J. Biomech. 33, 1089–1097 (2000).
Gao, C., Zhao, J. & Gregersen, H. Histomorphometry and strain distribution in pig duodenum with reference to zero-stress state. Dig. Dis. Sci. 45, 1500–1508 (2000).
Li, J., Zhao, J., Liao, D. & Gregersen, H. Effect of smooth muscle tone on morphometry and residual strain in rat duodenum, jejunum and ileum. J. Biomech. 41, 2667–2672 (2008).
Zhao, J., Liao, D., Chen, P., Kunwald, P. & Gregersen, H. Stomach stress and strain depend on location, direction and the layered structure. J. Biomech. 41, 3441–3447 (2008).
Benias, P. C. et al. Structure and distribution of an unrecognized interstitium in human tissues. Sci. Rep. 8, 4947 (2018).
Filzmayer, A. K. et al. Compression and stretch sensitive submucosal neurons of the porcine and human colon. Sci. Rep. 10, 13791 (2020).
Frieling, T., Wood, J. D. & Cooke, H. J. Submucosal reflexes: distension-evoked ion transport in the guinea pig distal colon. Am. J. Physiol. 263, G91–G96 (1992).
Brierley, S. M., Jones, R. C. 3rd, Gebhart, G. F. & Blackshaw, L. A. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127, 166–178 (2004).
Corsetti, M., Gevers, A. M., Caenepeel, P. & Tack, J. The role of tension receptors in colonic mechanosensitivity in humans. Gut 53, 1787–1793 (2004).
Oliver, K. M. et al. Molecular correlates of muscle spindle and Golgi tendon organ afferents. Nat. Commun. 12, 1451 (2021).
Iggo, A. Gastro-intestinal tension receptors with unmyelinated afferent fibres in the vagus of the cat. Q. J. Exp. Physiol. Cogn. Med. Sci. 42, 130–143 (1957).
Cannon, W. B. Peristalsis, segmentation, and the myenteric reflex. Am. J. Physiol. 30, 114–128 (1912).
Beyder, A. In pursuit of the epithelial mechanosensitivity mechanisms. Front. Endocrinol. 9, 804 (2018).
de Lorijn, F. et al. Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut 54, 1107–1113 (2005).
Synnerstad, I., Ekblad, E., Sundler, F. & Holm, L. Gastric mucosal smooth muscles may explain oscillations in glandular pressure: role of vasoactive intestinal peptide. Gastroenterology 114, 284–294 (1998).
Choe, K. et al. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J. Clin. Invest. 125, 4042–4052 (2015).
Alvarez, W. C. The myogenic nature of the rhythmic contractions of the intestine. Am. J. Physiol. Leg. Content 59, 421–430 (1922).
Dinning, P. G., Costa, M., Brookes, S. J. & Spencer, N. J. Neurogenic and myogenic motor patterns of rabbit proximal, mid, and distal colon. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G83–G92 (2012).
Dinning, P. G. et al. Neural mechanisms of peristalsis in the isolated rabbit distal colon: a neuromechanical loop hypothesis. Front. Neurosci. 8, 75 (2014).
Huizinga, J. D. et al. The origin of segmentation motor activity in the intestine. Nat. Commun. 5, 3326 (2014).
Cannon, W. B. The relation of tonus to antiperistalsis in the colon. Am. J. Physiol. Leg. Cont. 29, 238–249 (1911).
McIntyre, A., Vincent, R. M., Perkins, A. C. & Spiller, R. C. Effect of bran, ispaghula, and inert plastic particles on gastric emptying and small bowel transit in humans: the role of physical factors. Gut 40, 223–227 (1997).
Kim, H. J., Li, H., Collins, J. J. & Ingber, D. E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proc. Natl Acad. Sci. USA 113, E7–E15 (2016).
Dobnikar, L. et al. Disease-relevant transcriptional signatures identified in individual smooth muscle cells from healthy mouse vessels. Nat. Commun. 9, 4567 (2018).
Farrugia, G. et al. A mechanosensitive calcium channel in human intestinal smooth muscle cells. Gastroenterology 117, 900–905 (1999).
Beyder, A. et al. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J. Physiol. 588, 4969–4985 (2010).
Won, K. J., Sanders, K. M. & Ward, S. M. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc. Natl Acad. Sci. USA 102, 14913–14918 (2005).
Strege, P. R. et al. Sodium current in human intestinal interstitial cells of Cajal. Am. J. Physiol. Gastrointest. Liver Physiol. 285, G1111–G1121 (2003).
Komuro, T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J. Physiol. 576, 653–658 (2006).
Powley, T. L. et al. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol. Motil. 20, 69–79 (2008).
Sanders, K. M., Koh, S. D., Ro, S. & Ward, S. M. Regulation of gastrointestinal motility — insights from smooth muscle biology. Nat. Rev. Gastroenterol. Hepatol. 9, 633–645 (2012).
Kurahashi, M. et al. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J. Physiol. 589, 697–710 (2011).
Seguella, L. & Gulbransen, B. D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat. Rev. Gastroenterol. Hepatol. 18, 571–587 (2021).
Liñán-Rico, A. et al. Molecular signaling and dysfunction of the human reactive enteric glial cell phenotype. Inflamm. Bowel Dis. 22, 1812–1834 (2016).
Grubisic, V. et al. Enteric glia modulate macrophage phenotype and visceral sensitivity following inflammation. Cell Rep. 32, 108100 (2020).
Muller, P. A. et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 158, 300–313 (2014).
Kraichely, R. E., Strege, P. R., Sarr, M. G., Kendrick, M. L. & Farrugia, G. Lysophosphatidyl choline modulates mechanosensitive L-type Ca2+ current in circular smooth muscle cells from human jejunum. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G833–G839 (2009).
Luo, J. et al. TRPV4 channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity 49, 107–119.e4 (2018).
Feng, Y. et al. Mechanosensing drives acuity of alphabeta T-cell recognition. Proc. Natl Acad. Sci. USA 114, E8204–E8213 (2017).
Solis, A. G. et al. Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 573, 69–74 (2019).
Zhang, X. et al. Unraveling the mechanobiology of immune cells. Curr. Opin. Biotechnol. 66, 236–245 (2020).
Upadhyaya, A. Mechanosensing in the immune response. Semin. Cell Dev. Biol. 71, 137–145 (2017).
Zhu, C., Chen, W., Lou, J., Rittase, W. & Li, K. Mechanosensing through immunoreceptors. Nat. Immunol. 20, 1269–1278 (2019).
Michalick, L. & Kuebler, W. M. TRPV4-A missing link between mechanosensation and immunity. Front. Immunol. 11, 413 (2020).
Wu, P. et al. Mechano-regulation of peptide-MHC class I conformations determines TCR antigen recognition. Mol. Cell 73, 1015–1027 (2019).
Mazzuoli, G. & Schemann, M. Mechanosensitive enteric neurons in the myenteric plexus of the mouse intestine. PLoS ONE 7, e39887 (2012).
Mayer, C. J. & Wood, J. D. Properties of mechanosensitive neurons within Auerbach’s plexus of the small intestine of the cat. Pflug. Arch. 357, 35–49 (1975).
Mazzuoli-Weber, G. & Schemann, M. Mechanosensitive enteric neurons in the guinea pig gastric corpus. Front. Cell. Neurosci. 9, 430 (2015).
Mazzuoli, G. & Schemann, M. Multifunctional rapidly adapting mechanosensitive enteric neurons (RAMEN) in the myenteric plexus of the guinea pig ileum. J. Physiol. 587, 4681–4694 (2009).
Kugler, E. M. et al. Mechanical stress activates neurites and somata of myenteric neurons. Front. Cell Neurosci. 9, 342 (2015).
Mazzuoli-Weber, G. et al. Piezo proteins: incidence and abundance in the enteric nervous system. Is there a link with mechanosensitivity? Cell Tissue Res. 375, 605–618 (2019).
Mihara, H., Suzuki, N., Yamawaki, H., Tominaga, M. & Sugiyama, T. TRPV2 ion channels expressed in inhibitory motor neurons of gastric myenteric plexus contribute to gastric adaptive relaxation and gastric emptying in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G235–G240 (2013).
Dong, H., Tang, B., Jiang, Y. & Mittal, R. K. Na+/Ca2+ exchanger 1 is a key mechanosensitive molecule of the esophageal myenteric neurons. Acta Physiol. 225, e13223 (2019).
Bulbring, E. & Crema, A. The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa. J. Physiol. 146, 18–28 (1959).
Treichel, A. J., Farrugia, G. & Beyder, A. The touchy business of gastrointestinal (GI) mechanosensitivity. Brain Res. 1693, 197–200 (2018).
Alcaino, C. et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc. Natl Acad. Sci. USA 115, E7632–E7641 (2018).
Wang, F. et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol. 595, 79–91 (2017).
Billing, L. J. et al. Single cell transcriptomic profiling of large intestinal enteroendocrine cells in mice — identification of selective stimuli for insulin-like peptide-5 and glucagon-like peptide-1 co-expressing cells. Mol. Metab. 29, 158–169 (2019).
Linan-Rico, A. et al. Mechanosensory signaling in enterochromaffin cells and 5-HT release: potential implications for gut inflammation. Front. Neurosci. 10, 564 (2016).
Bertrand, P. P. Real-time measurement of serotonin release and motility in guinea pig ileum. J. Physiol. 577, 689–704 (2006).
Chin, A. et al. The role of mechanical forces and adenosine in the regulation of intestinal enterochromaffin cell serotonin secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G397–G405 (2012).
Treichel, A. J. et al. Specialized mechanosensory epithelial cells in mouse gut intrinsic tactile sensitivity. Gastroenterology https://doi.org/10.1053/j.gastro.2021.10.026 (2021).
Spencer, N. J. & Hu, H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 17, 338–351 (2020).
Furness, J. B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294 (2012).
Furness, J. B. & Stebbing, M. J. The first brain: species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol. Motil. https://doi.org/10.1111/nmo.13234 (2018).
Furness, J. B., Jones, C., Nurgali, K. & Clerc, N. Intrinsic primary afferent neurons and nerve circuits within the intestine. Prog. Neurobiol. 72, 143–164 (2004).
Melo, C. G. S. et al. Identification of intrinsic primary afferent neurons in mouse jejunum. Neurogastroenterol. Motil. 32, e13989 (2020).
Kunze, W. A., Clerc, N., Bertrand, P. P. & Furness, J. B. Contractile activity in intestinal muscle evokes action potential discharge in guinea-pig myenteric neurons. J. Physiol. 517, 547–561 (1999).
Kunze, W. A., Clerc, N., Furness, J. B. & Gola, M. The soma and neurites of primary afferent neurons in the guinea-pig intestine respond differentially to deformation. J. Physiol. 526, 375–385 (2000).
Mazzuoli-Weber, G. & Schemann, M. Mechanosensitivity in the enteric nervous system. Front. Cell. Neurosci. 9, 408 (2015).
Mao, Y., Wang, B. & Kunze, W. Characterization of myenteric sensory neurons in the mouse small intestine. J. Neurophysiol. 96, 998–1010 (2006).
Morarach, K. et al. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat. Neurosci. 24, 34–46 (2021).
Qi, Y. et al. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat. Commun. 6, 8512 (2015).
Brookes, S. J., Spencer, N. J., Costa, M. & Zagorodnyuk, V. P. Extrinsic primary afferent signalling in the gut. Nat. Rev. Gastroenterol. Hepatol. 10, 286–296 (2013).
Furness, J. B., Rivera, L. R., Cho, H.-J., Bravo, D. M. & Callaghan, B. The gut as a sensory organ. Nat. Rev. Gastroenterol. Hepatol. 10, 729–740 (2013).
Smith-Edwards, K. M. et al. Extrinsic primary afferent neurons link visceral pain to colon motility through a spinal reflex in mice. Gastroenterology 157, 522–536 e522 (2019).
Spencer, N. J., Kyloh, M. A., Travis, L. & Dodds, K. N. Sensory nerve endings arising from single spinal afferent neurons that innervate both circular muscle and myenteric ganglia in mouse colon: colon–brain axis. Cell Tissue Res. 381, 25–34 (2020).
Spencer, N. J., Melinda A., Kyloh, M. A., Travis, L. & Dodds, K. N. Identification of spinal afferent nerve endings in the colonic mucosa and submucosa that communicate directly with the spinal cord: the gut–brain axis. J. Comp. Neurol. 528, 1742–1753 (2020).
Hughes, P. A., Brierley, S. M., Young, R. L. & Blackshaw, L. A. Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J. Comp. Neurol. 500, 863–875 (2007).
Holzer, P. Acid-sensing ion channels in gastrointestinal function. Neuropharmacology 94, 72–79 (2015).
Page, A. J. et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 54, 1408–1415 (2005).
Sharif-Naeini, R. Role of mechanosensitive ion channels in the sensation of pain. J. Neural Transm. 127, 407–414 (2020).
Bai, L. et al. Genetic identification of vagal sensory neurons that control feeding. Cell 179, 1129–1143 e1123 (2019).
Williams, E. K. et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 166, 209–221 (2016).
Zimmerman, C. A. et al. A gut-to-brain signal of fluid osmolarity controls thirst satiation. Nature 568, 98–102 (2019).
Paintal, A. S. A study of gastric stretch receptors; their role in the peripheral mechanism of satiation of hunger and thirst. J. Physiol. 126, 255–270 (1954).
Zagorodnyuk, V. P., Chen, B. N. & Brookes, S. J. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J. Physiol. 534, 255–268 (2001).
Kim, D. Y. et al. A neural circuit mechanism for mechanosensory feedback control of ingestion. Nature 580, 376–380 (2020).
Berthoud, H. R., Patterson, L. M., Neumann, F. & Neuhuber, W. L. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat. Embryol. 195, 183–191 (1997).
Phillips, R. J. & Powley, T. L. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res. Rev. 34, 1–26 (2000).
Berthoud, H. R. & Powley, T. L. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J. Comp. Neurol. 319, 261–276 (1992).
Grundy, D., Blackshaw, L. A. & Hillsley, K. Role of 5-hydroxytryptamine in gastrointestinal chemosensitivity. Dig. Dis. Sci. 39, 44S–47S (1994).
Kirchgessner, A. L., Tamir, H. & Gershon, M. D. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J. Neurosci. 12, 235–248 (1992).
Maksimovic, S. et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617–621 (2014).
Chang, W. et al. Merkel disc is a serotonergic synapse in the epidermis for transmitting tactile signals in mammals. Proc. Natl Acad. Sci. USA 113, E5491–E5500 (2016).
Ranade, S. S. et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125 (2014).
Woo, S. H., Lumpkin, E. A. & Patapoutian, A. Merkel cells and neurons keep in touch. Trends Cell Biol. 25, 74–81 (2015).
Huber, A. R., Agostini-Vulaj, D., Drage, M. G. & Lemmon, J. W. Tactile corpuscle-like bodies (Wagner–Meissner corpuscles) of the colorectum: a series of 5 cases. Int. J. Surg. Pathol. 25, 684–687 (2017).
Page, A. J. & Blackshaw, L. A. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J. Physiol. 512, 907–916 (1998).
Bessou, P. & Perl, E. R. A movement receptor of the small intestine. J. Physiol. 182, 404–426 (1966).
Song, X. et al. Identification of medium/high-threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology 137, 274–284, e271 (2009).
Leek, B. F. Abdominal and pelvic visceral receptors. Br. Med. Bull. 33, 163–168 (1977).
Brunsden, A. M., Brookes, S. J., Bardhan, K. D. & Grundy, D. Mechanisms underlying mechanosensitivity of mesenteric afferent fibers to vascular flow. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G422–G428 (2007).
Malin, S. A., Christianson, J. A., Bielefeldt, K. & Davis, B. M. TPRV1 expression defines functionally distinct pelvic colon afferents. J. Neurosci. 29, 743–752 (2009).
Meehan, A. G. & Kreulen, D. L. A capsaicin-sensitive inhibitory reflex from the colon to mesenteric arteries in the guinea-pig. J. Physiol. 448, 153–159 (1992).
Sheehan, D. The afferent nerve supply of the mesentery and its significance in the causation of abdominal pain. J. Anat. 67, 233–249 (1933).
Szurszewski, J. H., Ermilov, L. G. & Miller, S. M. Prevertebral ganglia and intestinofugal afferent neurones. Gut 51 (Suppl. 1), i6–i10 (2002).
Weems, W. A. & Szurszewski, J. H. An intracellular analysis of some intrinsic factors controlling neural output from inferior mesenteric ganglion of guinea pigs. J. Neurophysiol. 41, 305–321 (1978).
Hibberd, T. J., Zagorodnyuk, V. P., Spencer, N. J. & Brookes, S. J. Identification and mechanosensitivity of viscerofugal neurons. Neuroscience 225, 118–129 (2012).
Gold, M. S. & Gebhart, G. F. Nociceptor sensitization in pain pathogenesis. Nat. Med. 16, 1248–1257 (2010).
Hebden, J. M., Blackshaw, P. E., Perkins, A. C., D’Amato, M. & Spiller, R. C. Small bowel transit of a bran meal residue in humans: sieving of solids from liquids and response to feeding. Gut 42, 685–689 (1998).
Hoffman, B. U. et al. Merkel cells activate sensory neural pathways through adrenergic synapses. Neuron 100, 1401–1413 e1406 (2018).
Pan, H. & Gershon, M. D. Activation of intrinsic afferent pathways in submucosal ganglia of the guinea pig small intestine. J. Neurosci. 20, 3295–3309 (2000).
Cooke, H. J., Sidhu, M. & Wang, Y. Z. 5-HT activates neural reflexes regulating secretion in the guinea-pig colon. Neurogastroenterol. Motil. 9, 181–186 (1997).
Kaelberer, M. M. et al. A gut-brain neural circuit for nutrient sensory transduction. Science 361, eaat5236 (2018).
Kaelberer, M. M., Rupprecht, L. E., Liu, W. W., Weng, P. & Bohorquez, D. V. Neuropod cells: the emerging biology of gut-brain sensory transduction. Annu. Rev. Neurosci. 43, 337–353 (2020).
Raiford, T. & Mulinos, M. G. The myenteric reflex as exhibited by the exteriorized colon of the dog. Am. J. Physiol. Leg. Content 110, 129–136 (1934).
Smith, T. K. & Furness, J. B. Reflex changes in circular muscle activity elicited by stroking the mucosa: an electrophysiological analysis in the isolated guinea-pig ileum. J. Autonomic Nerv. Syst. 25, 205–218 (1988).
Spencer, N. J. et al. By what mechanism does ondansetron inhibit colonic migrating motor complexes: does it require endogenous serotonin in the gut wall? Neurogastroenterol. Motil. 25, 677–685 (2013).
Delvalle, N. M. et al. Communication between enteric neurons, glia, and nociceptors underlies the effects of tachykinins on neuroinflammation. Cell Mol. Gastroenterol. Hepatol. 6, 321–344 (2018).
Cipriani, G. et al. Change in populations of macrophages promotes development of delayed gastric emptying in mice. Gastroenterology 154, 2122–2136 e2112 (2018).
Furness, J. B., Callaghan, B. P., Rivera, L. R. & Cho, H. J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 817, 39–71 (2014).
Lynn, P., Zagorodnyuk, V., Hennig, G., Costa, M. & Brookes, S. Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal gut. J. Physiol. 564, 589–601 (2005).
Romero, S., Le Clainche, C. & Gautreau, A. M. Actin polymerization downstream of integrins: signaling pathways and mechanotransduction. Biochem. J. 477, 1–21 (2020).
Zimmermann, D. & Kovar, D. R. Feeling the force: formin’s role in mechanotransduction. Curr. Opin. Cell Biol. 56, 130–140 (2019).
Fujiwara, S., Matsui, T. S., Ohashi, K., Mizuno, K. & Deguchi, S. Keratin-binding ability of the N-terminal Solo domain of Solo is critical for its function in cellular mechanotransduction. Genes. Cell 24, 390–402 (2019).
Schwayer, C. et al. Mechanosensation of tight junctions depends on ZO-1 phase separation and flow. Cell 179, 937–952 e918 (2019).
Kechagia, J. Z., Ivaska, J. & Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 20, 457–473 (2019).
Potla, R. et al. Molecular mapping of transmembrane mechanotransduction through the β1 integrin–CD98hc–TRPV4 axis. J. Cell Sci. 133, jcs248823 (2020).
Bachmann, M., Kukkurainen, S., Hytonen, V. P. & Wehrle-Haller, B. Cell adhesion by integrins. Physiol. Rev. 99, 1655–1699 (2019).
Ranade, S. S., Syeda, R. & Patapoutian, A. Mechanically activated ion channels. Neuron 87, 1162–1179 (2015).
Brierley, S. M. Molecular basis of mechanosensitivity. Auton. Neurosci. 153, 58–68 (2010).
Kraichely, R. E. & Farrugia, G. Mechanosensitive ion channels in interstitial cells of Cajal and smooth muscle of the gastrointestinal tract. Neurogastroenterol. Motil. 19, 245–252 (2007).
Arnadottir, J. & Chalfie, M. Eukaryotic mechanosensitive channels. Annu. Rev. Biophys. 39, 111–137 (2010).
Corey, D. et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432, 723–730 (2004).
Gottlieb, P. et al. Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflug. Arch. 455, 1097–1103 (2008).
Anishkin, A., Loukin, S. H., Teng, J. & Kung, C. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl Acad. Sci. USA 111, 7898–7905 (2014).
Lyford, G. L. et al. alpha(1C) (Ca(V)1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am. J. Physiol. Cell Physiol. 283, C1001–C1008 (2002).
Hao, J. et al. Kv1.1 channels act as mechanical brake in the senses of touch and pain. Neuron 77, 899–914 (2013).
Neshatian, L. et al. Ranolazine inhibits voltage-gated mechanosensitive sodium channels in human colon circular smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G506–G512 (2015).
Xu, J. GPR68 senses flow and is essential for vascular physiology. Cell 173, 762–775.e16 (2018).
Mederos y Schnitzler, M. et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 27, 3092–3103 (2008).
Bagal, S. K. et al. Ion channels as therapeutic targets: a drug discovery perspective. J. Med. Chem. 56, 593–624 (2013).
Bohorquez, D. V. et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Invest. 125, 782–786 (2015).
Hockley, J. R. F. et al. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68, 633–644 (2019).
Camunas-Soler, J. et al. Patch-Seq links single-cell transcriptomes to human islet dysfunction in diabetes. Cell Metab. 31, 1017–1031 e1014 (2020).
Cadwell, C. R. et al. Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat. Biotechnol. 34, 199–203 (2016).
Drokhlyansky, E. et al. The human and mouse enteric nervous system at single-cell resolution. Cell 182, 1606–1622 e1623 (2020).
Rakhilin, N. et al. An intravital window to image the colon in real time. Nat. Commun. 10, 5647 (2019).
Fosque, B. F. et al. Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators. Science 347, 755–760 (2015).
Moeyaert, B. et al. Improved methods for marking active neuron populations. Nat. Commun. 9, 4440 (2018).
Guo, J., Wang, Y., Sachs, F. & Meng, F. Actin stress in cell reprogramming. Proc. Natl Acad. Sci. USA 111, E5252–E5261 (2014).
Eisenstein, M. Mechanobiology: a measure of molecular muscle. Nature 544, 255–257 (2017).
Bozler, E. Myenteric reflex. Am. J. Physiol. 157, 329–337 (1949).
Major, G. et al. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology 152, 124–133 e122 (2017).
Bellono, N. W. et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170, 185–198 e116 (2017).
Schemann, M. & Mazzuoli, G. Multifunctional mechanosensitive neurons in the enteric nervous system. Auton. Neurosci. 153, 21–25 (2010).
Gregersen, H. & Kassab, G. Biomechanics of the gastrointestinal tract. Neurogastroenterol. Mot. 8, 277–297 (1996).
Vaishnav, R. N. & Vossoughi, J. Residual stress and strain in aortic segments. J. Biomech. 20, 235–239 (1987).
Acknowledgements
The authors thank the members of the Mayo Clinic Enteric NeuroScience Program (ENSP) group (G. Farrugia, J. H. Szurszewski, S. J. Gibbons and D. R. Linden), P. Gottlieb (SUNY, Buffalo, NJ, USA) for their constructive feedback, and L. Busby for administrative assistance. NIH support is acknowledged for GM065841, DK128913 (A.M.-P.), and DK052766, DK106456, DK100223 (A.B.).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks L. Ashley Blackshaw, Stuart Brierley and Hongzhen Hu for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Mechanosensors
-
Proteins directly involved in converting a mechanical stimulus into an intracellular electrochemical signal, for example, mechanogated ion channels that convert mechanical forces into an ionic flow.
- Signal amplification
-
Intracellular signalling steps between the primary signal generated by the mechanosensor and the final cellular output.
- Mechanotransducers
-
Proteins that amplify the signals generated by the mechanosensors and conduct the downstream cellular signalling in response to force.
- Non-specialized mechanosensory cells
-
Cells with a given non-mechanosensory primary function that use mechanosensors to sense mechanical stimuli and tune their own function in response to the physical state of their environment.
- Specialized mechanoreceptors
-
Cells whose primary function is the conversion of a mechanical stimulus into a physiological signal that influences the behaviour of other cells.
- Receptor current
-
Ionic current generated during sensory transduction, such as the opening of the mechanogated ion channels.
- Generator potentials
-
Transmembrane electrical potentials or voltage shifts due to receptor current that engage voltage-sensing transducer amplification elements.
Rights and permissions
About this article
Cite this article
Mercado-Perez, A., Beyder, A. Gut feelings: mechanosensing in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol 19, 283–296 (2022). https://doi.org/10.1038/s41575-021-00561-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-021-00561-y
This article is cited by
-
Modeling gut neuro-epithelial connections in a novel microfluidic device
Microsystems & Nanoengineering (2023)
-
Interoceptive rhythms in the brain
Nature Neuroscience (2023)
-
The gut microbiome and hypertension
Nature Reviews Nephrology (2023)
-
Immunoglobulin superfamily member 3 is required for the vagal neural crest cell migration and enteric neuronal network organization
Scientific Reports (2023)
-
Systemic sclerosis gastrointestinal dysmotility: risk factors, pathophysiology, diagnosis and management
Nature Reviews Rheumatology (2023)