Abstract
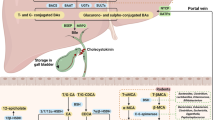
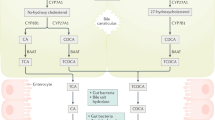
Farnesoid X receptor (FXR) is a ligand-activated transcription factor involved in the control of bile acid (BA) synthesis and enterohepatic circulation. FXR can influence glucose and lipid homeostasis. Hepatic FXR activation by obeticholic acid is currently used to treat primary biliary cholangitis. Late-stage clinical trials investigating the use of obeticholic acid in the treatment of nonalcoholic steatohepatitis are underway. Mouse models of metabolic disease have demonstrated that inhibition of intestinal FXR signalling reduces obesity, insulin resistance and fatty liver disease by modulation of hepatic and gut bacteria-mediated BA metabolism, and intestinal ceramide synthesis. FXR also has a role in the pathogenesis of gastrointestinal and liver cancers. Studies using tissue-specific and global Fxr-null mice have revealed that FXR acts as a suppressor of hepatocellular carcinoma, mainly through regulating BA homeostasis. Loss of whole-body FXR potentiates progression of spontaneous colorectal cancer, and obesity-induced BA imbalance promotes intestinal stem cell proliferation by suppressing intestinal FXR in Apcmin/+ mice. Owing to altered gut microbiota and FXR signalling, changes in overall BA levels and specific BA metabolites probably contribute to enterohepatic tumorigenesis. Modulating intestinal FXR signalling and altering BA metabolites are potential strategies for gastrointestinal and liver cancer prevention and treatment. In this Review, studies on the role of FXR in metabolic diseases and gastrointestinal and liver cancer are discussed, and the potential for development of targeted drugs are summarized.
Key points
-
Farnesoid X receptor (FXR) signalling in liver and intestine modulates enterohepatic bile acid circulation and lipid and glucose metabolism.
-
Both activation of hepatic FXR and inhibition of intestinal FXR have beneficial effects on obesity-related metabolic diseases.
-
As a transcriptional factor, FXR directly regulates expression of tumour suppressors involved in gastrointestinal and liver cancers.
-
The protective role of FXR in hepatocellular carcinoma mainly depends on hepatic modulation of bile acid homeostasis.
-
Tissue-specific FXR agonists and antagonists should be explored as potentially clinical drugs for metabolic disease and cancer.
-
Gut microbiota-derived bile acid metabolism should be considered as a new drug target for development of therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
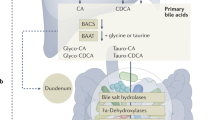
Russell, D. W. Fifty years of advances in bile acid synthesis and metabolism. J. Lipid Res. 50, S120–S125 (2009).
Chiang, J. Y. Bile acid metabolism and signaling. Compr. Physiol. 3, 1191–1212 (2013).
Wahlstrom, A., Sayin, S. I., Marschall, H. U. & Backhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50 (2016).
Hofmann, A. F. & Hagey, L. R. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J. Lipid Res. 55, 1553–1595 (2014).
Dawson, P. A. Hepatic bile acid uptake in humans and mice: multiple pathways and expanding potential role for gut-liver signaling. Hepatology 66, 1384–1386 (2017).
Matsubara, T., Li, F. & Gonzalez, F. J. FXR signaling in the enterohepatic system. Mol. Cell Endocrinol. 368, 17–29 (2013).
Forman, B. M. et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81, 687–693 (1995).
Makishima, M. et al. Identification of a nuclear receptor for bile acids. Science 284, 1362–1365 (1999).
Parks, D. J. et al. Bile acids: natural ligands for an orphan nuclear receptor. Science 284, 1365–1368 (1999).
Wang, H., Chen, J., Hollister, K., Sowers, L. C. & Forman, B. M. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol. Cell 3, 543–553 (1999).
Goodwin, B. et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 6, 517–526 (2000).
Kong, B. et al. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology 56, 1034–1043 (2012).
Denson, L. A. et al. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology 121, 140–147 (2001).
Ananthanarayanan, M., Balasubramanian, N., Makishima, M., Mangelsdorf, D. J. & Suchy, F. J. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J. Biol. Chem. 276, 28857–28865 (2001).
Sayin, S. I. et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235 (2013).
Sun, L. et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 24, 1919–1929 (2018).
Downes, M. et al. A chemical, genetic, and structural analysis of the nuclear bile acid receptor FXR. Mol. Cell 11, 1079–1092 (2003).
Pellicciari, R. et al. 6α-Ethyl-chenodeoxycholic acid (6-ECDCA), a potent and selective FXR agonist endowed with anticholestatic activity. J. Med. Chem. 45, 3569–3572 (2002).
Jiang, C. et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 6, 10166 (2015).
Gonzalez, F. J., Jiang, C. & Patterson, A. D. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151, 845–859 (2016).
Campbell, P. T. et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for US adults. Cancer Res. 76, 6076–6083 (2016).
Chen, Y., Wang, X., Wang, J., Yan, Z. & Luo, J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur. J. Cancer 48, 2137–2145 (2012).
Bardou, M., Barkun, A. N. & Martel, M. Obesity and colorectal cancer. Gut 62, 933–947 (2013).
Cariou, B. et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J. Biol. Chem. 281, 11039–11049 (2006).
Sinal, C. J. et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102, 731–744 (2000).
Prawitt, J. et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes 60, 1861–1871 (2011).
Zhang, Y. et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc. Natl Acad. Sci. USA 103, 1006–1011 (2006).
Hirschfield, G. M. et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 148, 751–761 e758 (2015).
Milona, A. et al. Raised hepatic bile acid concentrations during pregnancy in mice are associated with reduced farnesoid X receptor function. Hepatology 52, 1341–1349 (2010).
Hoofnagle, J. H. FXR agonists as therapy for liver disease. Hepatology 72, 1–3 (2020).
Markham, A. & Keam, S. J. Obeticholic acid: first global approval. Drugs 76, 1221–1226 (2016).
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2015).
Issa, D., Wattacheril, J. & Sanyal, A. J. Treatment options for nonalcoholic steatohepatitis–a safety evaluation. Expert Opin. Drug Saf. 16, 903–913 (2017).
Chapman, R. W. & Lynch, K. D. Obeticholic acid–a new therapy in PBC and NASH. Br. Med. Bull. 133, 95–104 (2020).
Zhang, S., Wang, J., Liu, Q. & Harnish, D. C. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J. Hepatol. 51, 380–388 (2009).
Zhou, J. et al. SUMOylation inhibitors synergize with FXR agonists in combating liver fibrosis. Nat. Commun. 11, 240 (2020).
Gonzalez, F. J. Nuclear receptor control of enterohepatic circulation. Compr. Physiol. 2, 2811–2828 (2012).
Xie, C. et al. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66, 613–626 (2017).
Chaurasia, B. & Summers, S. A. Ceramides - Lipotoxic inducers of metabolic disorders. Trends Endocrinol. Metab. 26, 538–550 (2015).
Kubota, K. et al. Improvements of mean body mass index and body weight in preobese and overweight Japanese adults with black Chinese tea (Pu-Erh) water extract. Nutr. Res. 31, 421–428 (2011).
Huang, F. et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 10, 4971 (2019).
Fang, S. et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 21, 159–165 (2015).
Pathak, P. et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology 68, 1574–1588 (2018).
Pathak, P. et al. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J. Biol. Chem. 292, 11055–11069 (2017).
Chiang, J. Y. L. & Ferrell, J. M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol. Gastrointest. Liver Physiol. 318, G554–G573 (2020).
Trabelsi, M. S. et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun. 6, 7629 (2015).
Ducastel, S. et al. The nuclear receptor FXR inhibits glucagon-like peptide-1 secretion in response to microbiota-derived short-chain fatty acids. Sci. Rep. 10, 174 (2020).
Keum, N. & Giovannucci, E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 16, 713–732 (2019).
Fodde, R., Smits, R. & Clevers, H. APC, signal transduction and genetic instability in colorectal cancer. Nat. Rev. Cancer 1, 55–67 (2001).
Lax, S. et al. Expression of the nuclear bile acid receptor/farnesoid X receptor is reduced in human colon carcinoma compared to nonneoplastic mucosa independent from site and may be associated with adverse prognosis. Int. J. Cancer 130, 2232–2239 (2012).
Modica, S. et al. The intestinal nuclear receptor signature with epithelial localization patterns and expression modulation in tumors. Gastroenterology 138, 636–648.E12 (2010).
De Gottardi, A. et al. The bile acid nuclear receptor FXR and the bile acid binding protein IBABP are differently expressed in colon cancer. Dig. Dis. Sci. 49, 982–989 (2004).
Selmin, O. I. et al. Inactivation of adenomatous polyposis coli reduces bile acid/farnesoid X receptor expression through Fxr gene CpG methylation in mouse colon tumors and human colon cancer cells. J. Nutr. 146, 236–242 (2016).
Bailey, A. M. et al. FXR silencing in human colon cancer by DNA methylation and KRAS signaling. Am. J. Physiol. Gastrointest. Liver Physiol. 306, G48–G58 (2014).
Modica, S. et al. Transcriptional regulation of the intestinal nuclear bile acid farnesoid X receptor (FXR) by the caudal-related homeobox 2 (CDX2). J. Biol. Chem. 289, 28421–28432 (2014).
Modica, S., Murzilli, S., Salvatore, L., Schmidt, D. R. & Moschetta, A. Nuclear bile acid receptor FXR protects against intestinal tumorigenesis. Cancer Res. 68, 9589–9594 (2008).
Maran, R. R. et al. Farnesoid X receptor deficiency in mice leads to increased intestinal epithelial cell proliferation and tumor development. J. Pharmacol. Exp. Ther. 328, 469–477 (2009).
Peng, Z., Raufman, J. P. & Xie, G. Src-mediated cross-talk between farnesoid X and epidermal growth factor receptors inhibits human intestinal cell proliferation and tumorigenesis. PLoS ONE 7, e48461 (2012).
Qiao, P., Li, S., Zhang, H., Yao, L. & Wang, F. Farnesoid X receptor inhibits proliferation of human colorectal cancer cells via the miR135A1/CCNG2 signaling pathway. Oncol. Rep. 40, 2067–2078 (2018).
Yu, J. et al. Farnesoid X receptor antagonizes Wnt/β-catenin signaling in colorectal tumorigenesis. Cell Death Dis. 11, 640 (2020).
Lee, Y. J. et al. The role of nuclear receptor subfamily 1 group H member 4 (NR1H4) in colon cancer cell survival through the regulation of c-Myc stability. Mol. Cell 43, 459–468 (2020).
Peng, Z., Chen, J., Drachenberg, C. B., Raufman, J. P. & Xie, G. Farnesoid X receptor represses matrix metalloproteinase 7 expression, revealing this regulatory axis as a promising therapeutic target in colon cancer. J. Biol. Chem. 294, 8529–8542 (2019).
Vavassori, P., Mencarelli, A., Renga, B., Distrutti, E. & Fiorucci, S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J. Immunol. 183, 6251–6261 (2009).
Gadaleta, R. M. et al. Fibroblast growth factor 19 modulates intestinal microbiota and inflammation in presence of farnesoid X receptor. EBioMedicine 54, 102719 (2020).
Costarelli, V. et al. A prospective study of serum bile acid concentrations and colorectal cancer risk in post-menopausal women on the island of Guernsey. Br. J. Cancer 86, 1741–1744 (2002).
Cao, H. et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int. J. Cancer 140, 2545–2556 (2017).
Bernstein, H., Bernstein, C., Payne, C. M., Dvorakova, K. & Garewal, H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat. Res. 589, 47–65 (2005).
Bernstein, C. et al. Carcinogenicity of deoxycholate, a secondary bile acid. Arch. Toxicol. 85, 863–871 (2011).
Kuhn, T. et al. Prediagnostic plasma bile acid levels and colon cancer risk: a prospective study. J. Natl Cancer Inst. 112, 516–524 (2020).
Dermadi, D. et al. Western diet deregulates bile acid homeostasis, cell proliferation, and tumorigenesis in colon. Cancer Res. 77, 3352–3363 (2017).
Fu, T. et al. FXR regulates intestinal cancer stem cell proliferation. Cell 176, 1098–1112 e1018 (2019).
Lew, J. L. et al. The farnesoid X receptor controls gene expression in a ligand- and promoter-selective fashion. J. Biol. Chem. 279, 8856–8861 (2004).
Souris, J. S. et al. A novel mouse model of sporadic colon cancer induced by combination of conditional Apc genes and chemical carcinogen in the absence of Cre recombinase. Carcinogenesis 40, 1376–1386 (2019).
Hinoi, T. et al. Mouse model of colonic adenoma-carcinoma progression based on somatic Apc inactivation. Cancer Res. 67, 9721–9730 (2007).
van de Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Campbell, C. et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 581, 475–479 (2020).
Kim, E. K., Cho, J. H., Kim, E. & Kim, Y. J. Ursodeoxycholic acid inhibits the proliferation of colon cancer cells by regulating oxidative stress and cancer stem-like cell growth. PLoS ONE 12, e0181183 (2017).
Khare, S. et al. Ursodeoxycholic acid suppresses Cox-2 expression in colon cancer: roles of Ras, p38, and CCAAT/enhancer-binding protein. Nutr. Cancer 60, 389–400 (2008).
Im, E. & Martinez, J. D. Ursodeoxycholic acid (UDCA) can inhibit deoxycholic acid (DCA)-induced apoptosis via modulation of EGFR/Raf-1/ERK signaling in human colon cancer cells. J. Nutr. 134, 483–486 (2004).
Shah, S. A., Volkov, Y., Arfin, Q., Abdel-Latif, M. M. & Kelleher, D. Ursodeoxycholic acid inhibits interleukin 1 beta [corrected] and deoxycholic acid-induced activation of NF-κB and AP-1 in human colon cancer cells. Int. J. Cancer 118, 532–539 (2006).
Liu, L., Fishman, M. L., Hicks, K. B., Kende, M. & Ruthel, G. Pectin/zein beads for potential colon-specific drug delivery: synthesis and in vitro evaluation. Drug Deliv. 13, 417–423 (2006).
Yan, F. et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Invest. 121, 2242–2253 (2011).
Amidon, S., Brown, J. E. & Dave, V. S. Colon-targeted oral drug delivery systems: design trends and approaches. AAPS PharmSciTech 16, 731–741 (2015).
El-Serag, H. B. & Rudolph, K. L. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132, 2557–2576 (2007).
Huang, X. F., Zhao, W. Y. & Huang, W. D. FXR and liver carcinogenesis. Acta Pharmacol. Sin. 36, 37–43 (2015).
Wolfe, A. et al. Increased activation of the Wnt/β-catenin pathway in spontaneous hepatocellular carcinoma observed in farnesoid X receptor knockout mice. J. Pharmacol. Exp. Ther. 338, 12–21 (2011).
Su, H. et al. Downregulation of nuclear receptor FXR is associated with multiple malignant clinicopathological characteristics in human hepatocellular carcinoma. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1245–G1253 (2012).
Kim, I. et al. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28, 940–946 (2007).
Yang, F. et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 67, 863–867 (2007).
Takahashi, S. et al. Role of farnesoid X receptor and bile acids in hepatic tumor development. Hepatol. Commun. 2, 1567–1582 (2018).
Liu, X. et al. Farnesoid X receptor associates with beta-catenin and inhibits its activity in hepatocellular carcinoma. Oncotarget 6, 4226–4238 (2015).
Kong, B. et al. Mice with hepatocyte-specific FXR deficiency are resistant to spontaneous but susceptible to cholic acid-induced hepatocarcinogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G295–G302 (2016).
Liang, Y., Yang, Z. & Zhong, R. Primary biliary cirrhosis and cancer risk: a systematic review and meta-analysis. Hepatology 56, 1409–1417 (2012).
Knisely, A. S. et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology 44, 478–486 (2006).
Cariello, M. et al. Long-term administration of nuclear bile acid receptor FXR agonist prevents spontaneous hepatocarcinogenesis in Abcb4(-/-) mice. Sci. Rep. 7, 11203 (2017).
Kimhofer, T., Fye, H., Taylor-Robinson, S., Thursz, M. & Holmes, E. Proteomic and metabonomic biomarkers for hepatocellular carcinoma: a comprehensive review. Br. J. Cancer 112, 1141–1156 (2015).
Liao, M. et al. Role of bile salt in regulating Mcl-1 phosphorylation and chemoresistance in hepatocellular carcinoma cells. Mol. Cancer 10, 44 (2011).
Anakk, S. et al. Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 5, 1060–1069 (2013).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Ma, C. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360 (2018).
Kong, B. et al. Fibroblast growth factor 15-dependent and bile acid-independent promotion of liver regeneration in mice. Hepatology 68, 1961–1976 (2018).
Li, Q. et al. The ileal FGF15/19 to hepatic FGFR4 axis regulates liver regeneration after partial hepatectomy in mice. J. Physiol. Biochem. 74, 247–260 (2018).
Fon Tacer, K. et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol. Endocrinol. 24, 2050–2064 (2010).
Zweers, S. J. et al. The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology 55, 575–583 (2012).
Schaap, F. G., van der Gaag, N. A., Gouma, D. J. & Jansen, P. L. High expression of the bile salt-homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 49, 1228–1235 (2009).
Sawey, E. T. et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by oncogenomic screening. Cancer Cell 19, 347–358 (2011).
Ahn, S. M. et al. Genomic portrait of resectable hepatocellular carcinomas: implications of RB1 and FGF19 aberrations for patient stratification. Hepatology 60, 1972–1982 (2014).
Zhao, H. et al. FGF19 promotes epithelial-mesenchymal transition in hepatocellular carcinoma cells by modulating the GSK3β/β-catenin signaling cascade via FGFR4 activation. Oncotarget 7, 13575–13586 (2016).
Chen, J. et al. Fibroblast growth factor 19-mediated up-regulation of SYR-related high-mobility group Box 18 promotes hepatocellular carcinoma metastasis by transactivating fibroblast growth factor receptor 4 and Fms-related tyrosine kinase 4. Hepatology 71, 1712–1731 (2020).
Uriarte, I. et al. Ileal FGF15 contributes to fibrosis-associated hepatocellular carcinoma development. Int. J. Cancer 136, 2469–2475 (2015).
Zhou, M. et al. Mouse species-specific control of hepatocarcinogenesis and metabolism by FGF19/FGF15. J. Hepatol. 66, 1182–1192 (2017).
Guo, F. et al. FXR induces SOCS3 and suppresses hepatocellular carcinoma. Oncotarget 6, 34606–34616 (2015).
Gao, B., Wang, H., Lafdil, F. & Feng, D. STAT proteins – key regulators of anti-viral responses, inflammation, and tumorigenesis in the liver. J. Hepatol. 57, 430–441 (2012).
Deuschle, U. et al. FXR controls the tumor suppressor NDRG2 and FXR agonists reduce liver tumor growth and metastasis in an orthotopic mouse xenograft model. PLoS ONE 7, e43044 (2012).
He, J. et al. Upregulation of microRNA-122 by farnesoid X receptor suppresses the growth of hepatocellular carcinoma cells. Mol. Cancer 14, 163 (2015).
Gadaleta, R. M. et al. Suppression of hepatic bile acid synthesis by a non-tumorigenic FGF19 analogue protects mice from fibrosis and hepatocarcinogenesis. Sci. Rep. 8, 17210 (2018).
Kang, H. J. et al. Characterization of hepatocellular carcinoma patients with FGF19 amplification assessed by fluorescence in situ hybridization: a large cohort study. Liver Cancer 8, 12–23 (2019).
Kim, R. D. et al. First-in-human phase I study of fisogatinib (BLU-554) validates aberrant FGF19 signaling as a driver event in hepatocellular carcinoma. Cancer Discov. 9, 1696–1707 (2019).
Attia, Y. M., Tawfiq, R. A., Ali, A. A. & Elmazar, M. M. The FXR agonist, obeticholic acid, suppresses HCC proliferation & metastasis: role of IL-6/STAT3 signalling pathway. Sci. Rep. 7, 12502 (2017).
Liu, T. et al. Mechanisms of MAFG dysregulation in cholestatic liver injury and development of liver cancer. Gastroenterology 155, 557–571.e14 (2018).
Al-Dury, S. et al. Obeticholic acid may increase the risk of gallstone formation in susceptible patients. J. Hepatol. 71, 986–991 (2019).
Mudaliar, S. et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 145, 574–582.e1 (2013).
Kanthan, R., Senger, J. L., Ahmed, S. & Kanthan, S. C. Gallbladder cancer in the 21st century. J. Oncol. 2015, 967472 (2015).
Obama, K. et al. Genome-wide analysis of gene expression in human intrahepatic cholangiocarcinoma. Hepatology 41, 1339–1348 (2005).
Zhong, X. Y. et al. MicroRNA-421 functions as an oncogenic miRNA in biliary tract cancer through down-regulating farnesoid X receptor expression. Gene 493, 44–51 (2012).
Wang, W. et al. FXR agonists enhance the sensitivity of biliary tract cancer cells to cisplatin via SHP dependent inhibition of Bcl-xL expression. Oncotarget 7, 34617–34629 (2016).
Zuo, M. et al. RNA sequencing-based analysis of gallbladder cancer reveals the importance of the liver X receptor and lipid metabolism in gallbladder cancer. Oncotarget 7, 35302–35312 (2016).
Erice, O. et al. Differential effects of FXR or TGR5 activation in cholangiocarcinoma progression. Biochim. Biophy. Acta Mol. Basis Dis. 1864, 1335–1344 (2018).
Di Matteo, S. et al. The FXR agonist obeticholic acid inhibits the cancerogenic potential of human cholangiocarcinoma. PLoS ONE 14, e0210077 (2019).
Lozano, E. et al. Cocarcinogenic effects of intrahepatic bile acid accumulation in cholangiocarcinoma development. Mol. Cancer Res. 12, 91–100 (2014).
Gege, C. et al. Ligands: current status and clinical applications. Handb. Exp. Pharmacol. 256, 167–205 (2019).
Yamada, S. et al. Bile acid metabolism regulated by the gut microbiota promotes non-alcoholic steatohepatitis-associated hepatocellular carcinoma in mice. Oncotarget 9, 9925–9939 (2018).
Wirbel, J. et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 25, 679–689 (2019).
Chen, F. et al. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J. Biol. Chem. 278, 19909–19916 (2003).
Frankenberg, T. et al. Regulation of the mouse organic solute transporter α-β, Ostα-Ostβ, by bile acids. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G912–G922 (2006).
Inagaki, T. et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2, 217–225 (2005).
Grober, J. et al. Identification of a bile acid-responsive element in the human ileal bile acid-binding protein gene. Involvement of the farnesoid X receptor/9-cis-retinoic acid receptor heterodimer. J. Biol. Chem. 274, 29749–29754 (1999).
Zollner, G., Marschall, H. U., Wagner, M. & Trauner, M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol. Pharm. 3, 231–251 (2006).
Van Mil, S. W. et al. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology 133, 507–516 (2007).
Yang, Z. X., Shen, W. & Sun, H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol. Int. 4, 741–748 (2010).
Takahashi, S. et al. Farnesoid X receptor protects against low-dose carbon tetrachloride-induced liver injury through the taurocholate-JNK pathway. Toxicol. Sci. 158, 334–346 (2017).
Fiorucci, S. et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology 127, 1497–1512 (2004).
Fiorucci, S. et al. A farnesoid X receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J. Pharmacol. Exp. Ther. 314, 584–595 (2009).
Wu, W. B. et al. Activation of farnesoid X receptor attenuates hepatic injury in a murine model of alcoholic liver disease. Biochem. Bioph Res. Co. 443, 68–73 (2014).
Manley, S. et al. Farnesoid X receptor regulates forkhead Box O3a activation in ethanol-induced autophagy and hepatotoxicity. Redox Biol. 2, 991–1002 (2014).
Lu, W. et al. FXR antagonism of NSAIDs contributes to drug-induced liver injury identified by systems pharmacology approach. Sci. Rep. 5, 8114 (2015).
Chen, W. D., Wang, Y. D., Meng, Z., Zhang, L. & Huang, W. Nuclear bile acid receptor FXR in the hepatic regeneration. Biochim. Biophys. Acta 1812, 888–892 (2011).
Gadaleta, R. M. et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60, 463–472 (2011).
Jiang, C. T. et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest. 125, 386–402 (2015).
Inagaki, T. et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl Acad. Sci. USA 103, 3920–3925 (2006).
Jiang, T. et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes 56, 2485–2493 (2007).
Gai, Z. et al. Farnesoid X receptor activation protects the kidney from ischemia-reperfusion damage. Sci. Rep. 7, 9815 (2017).
Zhao, K. et al. Activation of FXR protects against renal fibrosis via suppressing Smad3 expression. Sci. Rep. 6, 37234 (2016).
Rizzo, G. et al. The farnesoid X receptor promotes adipocyte differentiation and regulates adipose cell function in vivo. Mol. Pharmacol. 70, 1164–1173 (2006).
Nijmeijer, R. M. et al. Impact of global Fxr deficiency on experimental acute pancreatitis and genetic variation in the FXR locus in human acute pancreatitis. PLoS ONE 9, e114393 (2014).
Popescu, I. R. et al. The nuclear receptor FXR is expressed in pancreatic β-cells and protects human islets from lipotoxicity. FEBS Lett. 584, 2845–2851 (2010).
Moris, D., Giaginis, C., Tsourouflis, G. & Theocharis, S. Farnesoid-X receptor (FXR) as a promising pharmaceutical target in atherosclerosis. Curr. Med. Chem. 24, 1147–1157 (2017).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT04270682 (2020).
Liu, Y. P. et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J. Clin. Invest. 112, 1678–1687 (2003).
Younossi, Z. M. et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 394, 2184–2196 (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02855164 (2020).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02516605 (2019).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT02913105 (2021).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT03804879 (2020)
Al-Khaifi, A., Rudling, M. & Angelin, B. An FXR agonist reduces bile acid synthesis independently of increases in FGF19 in healthy volunteers. Gastroenterology 155, 1012–1016 (2018).
US National Library of Medicine. ClinicalTrials.gov https://ClinicalTrials.gov/show/NCT01999101 (2016).
Patel, K. et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology 72, 58–71 (2020).
Floreani, A. & Mangini, C. Primary biliary cholangitis: old and novel therapy. Eur. J. Intern. Med. 47, 1–5 (2018).
Acknowledgements
The authors are supported by the National Cancer Institute Intramural Research Program. J.C. was supported by a fellowship from the China Scholarship Council. We thank Y. Luo, S. Takahashi and T. Yan for reading the manuscript and providing feedback.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all the aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, L., Cai, J. & Gonzalez, F.J. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat Rev Gastroenterol Hepatol 18, 335–347 (2021). https://doi.org/10.1038/s41575-020-00404-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-020-00404-2
This article is cited by
-
The novel molecular mechanism of pulmonary fibrosis: insight into lipid metabolism from reanalysis of single-cell RNA-seq databases
Lipids in Health and Disease (2024)
-
Farnesoid X receptor promotes non-small cell lung cancer metastasis by activating Jak2/STAT3 signaling via transactivation of IL-6ST and IL-6 genes
Cell Death & Disease (2024)
-
Progress in gut microbiota-host interaction
Science China Life Sciences (2024)
-
Comprehensive insights: unraveling the mechanisms of gut commensals in glucose metabolism regulation
Science China Life Sciences (2024)
-
Integrated models of blood protein and metabolite enhance the diagnostic accuracy for Non-Small Cell Lung Cancer
Biomarker Research (2023)