Abstract
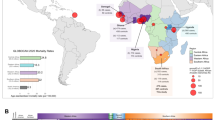
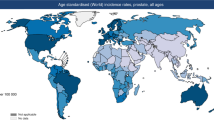
Prostate cancer is a global health problem, but incidence varies considerably across different continents. Asia is traditionally considered a low-incidence area, but the incidence and mortality of prostate cancer have rapidly increased across the continent. Substantial differences in epidemiological features have been observed among different Asian regions, and incidence, as well as mortality-to-incidence ratio, is associated with the human development index. Prostate cancer mortality decreased in Japan and Israel from 2007 to 2016, but mortality has increased in Thailand, Kyrgyzstan and Uzbekistan over the same period. Genomic analyses have shown a low prevalence of ERG oncoprotein in the East Asian population, alongside a low rate of PTEN loss, high CHD1 enrichments and high FOXA1 alterations. Contributions from single-nucleotide polymorphisms to prostate cancer risk vary with ethnicity, but germline mutation rates of DNA damage repair genes in metastatic prostate cancer are comparable in Chinese and white patients from the USA and UK. Pharmacogenomic features of testosterone metabolism might contribute to disparities seen in the response to androgen deprivation between East Asian men and white American and European men. Overall, considerable diversity in epidemiology and genomics of prostate cancer across Asia defines disease characteristics in these populations, but studies in this area are under-represented in the literature. Taking into account this intracontinental and intercontinental heterogeneity, translational studies are required in order to develop ethnicity-specific treatment strategies.
Key points
-
The epidemiology and genomic features of prostate cancer vary considerably between Asian regions and countries and genomic data are scarce.
-
Prostate cancer incidence is still increasing in Asia; however, from 2007 to 2016, mortality has decreased in highly developed countries such as Japan and Israel but increased in developing countries such as Thailand, Kyrgyzstan and Uzbekistan.
-
PSA screening is not common in Asian countries, but evidence from Japan, where a screening programme has been recommended by the Japanese Urological Association since 2008, showed that screening decreased the incidence of metastatic disease from 21.3% in 2000 to 11.6% in 2014 and is associated with improved survival.
-
Rates of ERG oncoprotein-positive prostate cancer are low (13–22%) in East Asian men, whereas FOXA1 is more frequently (41%) mutated in Chinese men with localized prostate cancer than in white patients.
-
Genetic studies have revealed remarkably different gene polymorphisms in men with prostate cancer of East Asian and European descent, whereas the rate of germline mutations in DNA damage repair genes was comparable (12%) in these populations.
-
Pharmacogenomic studies of testosterone metabolism-related genes have revealed an inherent difference in testosterone metabolism between East Asian men and white populations from the USA and Europe, providing a potential explanation for differences observed in the efficacy of androgen deprivation therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. https://doi.org/10.3322/caac.21660 (2021).
Pernar, C. H., Ebot, E. M., Wilson, K. M. & Mucci, L. A. The epidemiology of prostate cancer. Cold Spring Harb. Perspect. Med. 8, a030361 (2018).
Liu, Z. et al. Future of cancer incidence in Shanghai, China: predicting the burden upon the ageing population. Cancer Epidemiol. 60, 8–15 (2019).
Shin, S. et al. Dietary patterns and prostate cancer risk in Japanese: the Japan Public Health Center-based Prospective Study (JPHC Study). Cancer Causes Control. 29, 589–600 (2018).
Culp, M. B., Soerjomataram, I., Efstathiou, J. A., Bray, F. & Jemal, A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol. 77, 38–52 (2020).
Dai, C., Heemers, H. & Sharifi, N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. 7, a03045 (2017).
Ku, S. Y., Gleave, M. E. & Beltran, H. Towards precision oncology in advanced prostate cancer. Nat. Rev. Urol. 16, 645–654 (2019).
de Bono, J. et al. Olaparib for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 382, 2091–2102 (2020).
Abida, W. et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 5, 471–478 (2019).
Spratt, D. E. et al. Racial/ethnic disparities in genomic sequencing. JAMA Oncol. 2, 1070–1074 (2016).
Wang, Y., Lu, D., Chung, Y.-J. & Xu, S. Genetic structure, divergence and admixture of Han Chinese, Japanese and Korean populations. Hereditas 155, 19 (2018).
The United Nations Development Programme. Human Development Report 2019. United Nations Development Programme http://hdr.undp.org/sites/default/files/hdr2019.pdf (2019).
Goodarzi, E., Khazaei, Z., Sohrabivafa, M., Momenabadi, V. & Moayed, L. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide prostate cancers and their relationship with the human development index. Adv. Human Biol. 9, 245–250 (2019).
United Nations, Department of Economic and Social Affairs, Population Division. World Population Ageing 2019 (ST/ESA/SER.A/444) (2020).
United Nations Division. Methodology—Standard Country or Area Codes for Statistical Use (M49). United Nations https://unstats.un.org/unsd/methodology/m49/#geo-regions (2021).
Fidler, M. M., Soerjomataram, I. & Bray, F. A global view on cancer incidence and national levels of the human development index. Int. J. Cancer 139, 2436–2446 (2016).
Wong, M. C. et al. Global incidence and mortality for prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur. Urol. 70, 862–874 (2016).
The United Nations Development Programme. Human Development Report 2020. United Nations Development Programme http://hdr.undp.org/sites/default/files/hdr2020.pdf (2020).
Cutler, D., Huang, W. & Lleras-Muney, A. Economic conditions and mortality: evidence from 200 years of data (National Bureau of Economic Research, 2016).
Wang, S.-C. et al. Limited improvement in prostate cancer mortality-to-incidence ratios in countries with high health care expenditures. Aging 12, 21308–21315 (2020).
Fullman, N. et al. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet 391, 2236–2271 (2018).
Fowke, J. H. et al. Associations of body mass index, smoking, and alcohol consumption with prostate cancer mortality in the asia cohort consortium. Am. J. Epidemiol. 182, 381–389 (2015).
Ferlay, J., Colombet, M. & Bray, F. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase No. 9. IARC http://ci5.iarc.fr (2018).
World Health Organization. Department of information, evidence and research, mortality database. WHO https://www-dep.iarc.fr/WHOdb/WHOdb.htm (2019).
Kim, H. J., Fay, M. P., Feuer, E. J. & Midthune, D. N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 19, 335–351 (2000).
Ito, K., Kakehi, Y., Naito, S., Okuyama, A. & Japanese Urological Association, Japanese Urological Association guidelines on prostate-specific antigen-based screening for prostate cancer and the ongoing cluster cohort study in Japan. Int. J. Urol. 15, 763–768 (2008).
Zhang, J., Dhakal, I. B., Zhao, Z. & Li, L. Trends in mortality from cancers of the breast, colon, prostate, esophagus, and stomach in East Asia. Eur. J. Cancer Prev. 21, 480–489 (2012).
Alvarez, C. S. et al. Current and future burden of prostate cancer in Songkhla, Thailand: analysis of incidence and mortality trends from 1990 to 2030. J. Global Oncol. 4, 1–11 (2018).
Chiong, E. et al. Management of patients with advanced prostate cancer in the Asia Pacific region: ‘real-world’ consideration of results from the Advanced Prostate Cancer Consensus Conference (APCCC) 2017. BJU Int. 123, 22–34 (2019).
Koo, K. C., Lee, K. S. & Chung, B. H. Urologic cancers in Korea. Jpn. J. Clin. Oncol. 45, 805–811 (2015).
Ha Chung, B., Horie, S. & Chiong, E. The incidence, mortality, and risk factors of prostate cancer in Asian men. Prostate Int. 7, 1–8 (2019).
Genkinger, J. M. et al. Measures of body fatness and height in early and mid-to-late adulthood and prostate cancer: risk and mortality in the pooling project of prospective studies of diet and cancer. Ann. Oncol. 31, 103–114 (2020).
Gansler, T. et al. Smoking and prostate cancer–specific mortality after diagnosis in a large prospective cohort. Cancer Epidemiol. Biomarkers Prev. 27, 665–672 (2018).
Ng, S. W., Howard, A. G., Wang, H. J., Su, C. & Zhang, B. The physical activity transition among adults in China: 1991–2011. Obes. Rev. 15, 27–36 (2014).
Huang, L. et al. Nutrition transition and related health challenges over decades in China. Eur. J. Clin. Nutr. 75, 247–252 (2020).
Chen, Y. et al. The prevalence and increasing trends of overweight, general obesity, and abdominal obesity among Chinese adults: a repeated cross-sectional study. BMC Public Health 19, 1293 (2019).
Chen, W. et al. Cancer statistics in China, 2015. CA A Cancer J. Clin. 66, 115–132 (2016).
Hugosson, J. et al. A 16-yr follow-up of the european randomized study of screening for prostate cancer. Eur. Urol. 76, 43–51 (2019).
Ito, K. et al. Screening for prostate cancer: history, evidence, controversies and future perspectives toward individualized screening. Int. J. Urol. 26, 956–970 (2019).
Kakehi, Y., Sugimoto, M., Taoka, R. & Committee for establishment of the evidenced-based clinical practice guideline for prostate cancer of the Japanese Urological Association. Evidenced-based clinical practice guideline for prostate cancer (summary: Japanese Urological Association, 2016 edition). Int. J. Urol. 24, 648–666 (2017).
Kitagawa, Y. & Namiki, M. Prostate-specific antigen-based population screening for prostate cancer: current status in Japan and future perspective in Asia. Asian J. Androl. 17, 475–480 (2015).
Cancer Registration Committee of the Japanese Urological Association. Clinicopathological statistics on registered prostate cancer patients in Japan: 2000 report from the Japanese Urological Association. Int. J. Urol. 12, 46–61 (2005).
Fujimoto, H. et al. Oncological outcomes of the prostate cancer patients registered in 2004: Report from the Cancer Registration Committee of the JUA. Int. J. Urol. 18, 876–881 (2011).
Chen, R. et al. Prostate cancer in Asia: a collaborative report. Asian J. Urol. 1, 15–29 (2014).
Gandaglia, G. et al. Structured population-based prostate-specific antigen screening for prostate cancer: the European Association of Urology Position in 2019. Eur. Urol. 76, 142–150 (2019).
Yip, W. in Oxford Research Encyclopedia of Economics and Finance (Oxford University Press, 2019).
Bai, L. et al. Health care utilization and costs of patients with prostate cancer in China based on National Health Insurance Database from 2015 to 2017. Front. Pharmacol. 11, 719 (2020).
Roth, J. A., Gulati, R., Gore, J. L., Cooperberg, M. R. & Etzioni, R. Economic analysis of prostate-specific antigen screening and selective treatment strategies. JAMA Oncol. 2, 890–898 (2016).
Hinotsu, S., Namiki, M., Ozono, S. & Akaza, H. NCCN Asia Consensus Statement prostate cancer. Jpn. J. Clin. Oncol. 48, 964–965 (2018).
Hamashima, C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn. J. Clin. Oncol. 48, 278–286 (2018).
Insamran, W. & Sangrajrang, S. National cancer control program of Thailand. Asian Pac. J. Cancer Prev. 21, 577–582 (2020).
Kim, H. B. & Lee, S.-M. When public health intervention is not successful: cost sharing, crowd-out, and selection in Korea’s National Cancer Screening Program. J. Health Econ. 53, 100–116 (2017).
Coleman, M. P. et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 9, 730–756 (2008).
Allemani, C. et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 385, 977–1010 (2015).
Allemani, C. et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391, 1023–1075 (2018).
Committee for Establishment of the Guidelines on Screening for Prostate Cancer; Japanese Urological Association. Updated Japanese Urological Association Guidelines on prostate-specific antigen-based screening for prostate cancer in 2010. Int. J. Urol. 17, 830–838 (2010).
Tabuchi, T. et al. Determinants of participation in prostate cancer screening: a simple analytical framework to account for healthy-user bias. Cancer Sci. 106, 108–114 (2014).
Korea Central Cancer Registry. Annual Report of Cancer Statistics in Korea in 2016. Korea Central Cancer Registry https://ncc.re.kr/downloadByFileUrl.ncc?path=/downloadFiles/eng/Annual_report_of_cancer_statistics_in_Korea_in_2016.pdf (2018).
Singapore Cancer Registry Singapore Cancer Registry 50th Anniversary Monograph 1968–2017. Health Promotion Board https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library/thespore-cancerregistry_commerativebook_-1.pdf?sfvrsn=231fce6e_0 (2019).
Feinstein, A. R., Sosin, D. M. & Wells, C. K. The Will Rogers phenomenon. N. Engl. J. Med. 312, 1604–1608 (1985).
Park, J., Suh, B., Shin, D. W., Hong, J. H. & Ahn, H. Changing patterns of primary treatment in Korean men with prostate cancer over 10 years: a nationwide population based study. Cancer Res. Treat. 48, 899–906 (2016).
Carlsson, S. V. & Albertsen, P. C. Better survival after curative treatment for screen-detected prostate cancer compared with clinical diagnosis: a real effect or lead-time bias? Eur. Urol. 68, 183–184 (2015).
Soontrapa, S., Tantiwong, A., Leewansangtong, S. & Bhanalaph, T. Five-year follow-up of prostate cancer in Siriraj Hospital. J. Med. Assoc. Thai 83, 236–242 (2000).
Draisma, G. et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. JNCI J. Natl Cancer Inst. 101, 374–383 (2009).
Cook, L. S., Goldoft, M., Schwartz, S. M. & Weiss, N. S. Incidence of adenocarcinoma of the prostate in Asian immigrants to the United States and their descendants. J. Urol. 161, 152–155 (1999).
Kimura, T. et al. Time trends in histological features of latent prostate cancer in Japan. J. Urol. 195, 1415–1420 (2016).
Lichtensztajn, D. Y. et al. Prostate cancer risk profiles of asian-american men: disentangling the effects of immigration status and race/ethnicity. J. Urol. 191, 952–956 (2014).
Deuker, M. et al. PSA, stage, grade and prostate cancer specific mortality in Asian American patients relative to Caucasians according to the United States Census Bureau race definitions. World J. Urol. https://doi.org/10.1007/s00345-020-03242-8 (2020).
Darst, B. F. et al. The four-kallikrein panel is effective in identifying aggressive prostate cancer in a multiethnic population. Cancer Epidemiol. Biomarkers Prev. 29, 1381–1388 (2020).
Creed, J. H. et al. Commercial gene expression tests for prostate cancer prognosis provide paradoxical estimates of race-specific risk. Cancer Epidemiol. Biomarkers Prev. 29, 246–253 (2020).
Yee, W. W. et al. 218MO comparison of a 22-gene genomic classifier (GC) with NCCN for risk stratification of Asian prostate cancers (PCa). Ann. Oncol. 31, S1325–S1326 (2020).
Schroder, F. H. et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet 384, 2027–2035 (2014).
Garcia-Closas, M. & Berrington de Gonzalez, A. Invited commentary: screening and the elusive etiology of prostate cancer. Am. J. Epidemiol. 182, 390–393 (2015).
Li, J. et al. A genomic and epigenomic atlas of prostate cancer in Asian populations. Nature 580, 93–99 (2020).
Wang, G., Zhao, D., Spring, D. J. & DePinho, R. A. Genetics and biology of prostate cancer. Genes Dev. 32, 1105–1140 (2018).
Bhatia, V. & Ateeq, B. Molecular underpinnings governing genetic complexity of ETS-fusion-negative prostate cancer. Trends Mol. Med. 25, 1024–1038 (2019).
Pederzoli, F. et al. Targetable gene fusions and aberrations in genitourinary oncology. Nat. Rev. Urol. 17, 613–625 (2020).
Sedarsky, J., Degon, M., Srivastava, S. & Dobi, A. Ethnicity and ERG frequency in prostate cancer. Nat. Rev. Urol. 15, 125–131 (2018).
Shi, Z. et al. Biomarker analysis of the phase III IPATential150 trial of first-line ipatasertib (Ipat) plus abiraterone (Abi) in metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 38, 182–182 (2020).
Koide, H. et al. Comparison of ERG and SPINK1 expression among incidental and metastatic prostate cancer in Japanese men. Prostate 79, 3–8 (2019).
Khani, F. et al. Evidence for molecular differences in prostate cancer between African American and Caucasian men. Clin. Cancer Res. 20, 4925–4934 (2014).
Kong, D. P. et al. Prevalence and clinical application of TMPRSS2-ERG fusion in Asian prostate cancer patients: a large-sample study in Chinese people and a systematic review. Asian J. Androl. 2, 200–207 (2019).
Tan, J. S. J. et al. Heterogenous expression of ERG oncoprotein in Malaysian men with adenocarcinoma of the prostate. Malays. J. Pathol. 40, 103–110 (2018).
Pernar, C. H. et al. A prospective study of the association between physical activity and risk of prostate cancer defined by clinical features and TMPRSS2:ERG. Eur. Urol. 76, 33–40 (2019).
Graff, R. E. et al. Height, obesity, and the risk of TMPRSS2:ERG-defined prostate cancer. Cancer Epidemiol. Biomarkers Prev. 27, 193–200 (2018).
Nie, L. et al. The expression profile and heterogeneity analysis of ERG in 633 consecutive prostate cancers from a single center. Prostate 79, 819–825 (2019).
Graff, R. E. et al. The TMPRSS2:ERG fusion and response to androgen deprivation therapy for prostate cancer. Prostate 75, 897–906 (2015).
Lotan, T. L. et al. PTEN loss detection in prostate cancer: comparison of PTEN immunohistochemistry and PTEN FISH in a large retrospective prostatectomy cohort. Oncotarget 8, 65566–65576 (2017).
Mao, X. et al. Distinct genomic alterations in prostate cancers in Chinese and Western populations suggest alternative pathways of prostate carcinogenesis. Cancer Res. 70, 5207–5212 (2010).
de Bono, J. S. et al. Randomized phase II study evaluating akt blockade with ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin. Cancer Res. 25, 928–936 (2019).
Schoenborn, J. R., Nelson, P. & Fang, M. Genomic profiling defines subtypes of prostate cancer with the potential for therapeutic stratification. Clin. Cancer Res. 19, 4058–4066 (2013).
Kari, V. et al. Loss of CHD1 causes DNA repair defects and enhances prostate cancer therapeutic responsiveness. EMBO Rep. 17, 1609–1623 (2016).
Wang, C. et al. Serine protease inhibitor Kazal type 1 promotes epithelial-mesenchymal transition through EGFR signaling pathway in prostate cancer. Prostate 74, 689–701 (2014).
Pan, X. et al. The expression profile and prognostic value of SPINK1 in initially diagnosed bone metastatic prostate cancer. Prostate 76, 823–833 (2016).
Tomlins, S. A. et al. Characterization of 1577 primary prostate cancers reveals novel biological and clinicopathologic insights into molecular subtypes. Eur. Urol. 68, 555–567 (2015).
Wang, Z. et al. The diverse roles of SPOP in prostate cancer and kidney cancer. Nat. Rev. Urol. 17, 339–350 (2020).
Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015).
Augello, M. A. et al. CHD1 loss alters AR binding at lineage-specific enhancers and modulates distinct transcriptional programs to drive prostate tumorigenesis. Cancer Cell 35, 817–819 (2019).
Ren, S. et al. Whole-genome and transcriptome sequencing of prostate cancer identify new genetic alterations driving disease progression. Eur. Urol. 73, 322–339 (2017).
Shenoy, T. R. et al. CHD1 loss sensitizes prostate cancer to DNA damaging therapy by promoting error-prone double-strand break repair. Ann. Oncol. 28, 1495–1507 (2017).
Teng, M., Zhou, S., Cai, C., Lupien, M. & He, H. H. Pioneer of prostate cancer: past, present and the future of FOXA1. Protein Cell 12, 29–38 (2020).
Adams, E. J. et al. FOXA1 mutations alter pioneering activity, differentiation and prostate cancer phenotypes. Nature 571, 408–412 (2019).
Parolia, A. et al. Distinct structural classes of activating FOXA1 alterations in advanced prostate cancer. Nature 571, 413–418 (2019).
Liu, X. et al. Genetic alterations in esophageal tissues from squamous dysplasia to carcinoma. Gastroenterology 153, 166–177 (2017).
Jurcak, N. R. et al. Axon guidance molecules promote perineural invasion and metastasis of orthotopic pancreatic tumors in mice. Gastroenterology 157, 838–850 e836 (2019).
Hearn, J. W. D. et al. HSD3B1 genotype and clinical outcomes in metastatic castration-sensitive prostate cancer. JAMA Oncol. 6, e196496 (2020).
Castro, E. et al. PROREPAIR-B: a prospective cohort study of the impact of germline DNA repair mutations on the outcomes of patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 37, 490–503 (2019).
Sandhu, S. K. et al. PROfound: phase III study of olaparib versus enzalutamide or abiraterone for metastatic castration-resistant prostate cancer (mCRPC) with homologous recombination repair (HRR) gene alterations. Ann. Oncol. 30, ix188–ix189 (2019).
Gurdasani, D., Barroso, I., Zeggini, E. & Sandhu, M. S. Genomics of disease risk in globally diverse populations. Nat. Rev. Genet. 20, 520–535 (2019).
Wojcik, G. L. et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature 570, 514–518 (2019).
Farashi, S., Kryza, T., Clements, J. & Batra, J. Post-GWAS in prostate cancer: from genetic association to biological contribution. Nat. Rev. Cancer 19, 46–59 (2018).
Benafif, S., Kote-Jarai, Z. & Eeles, R. A. A review of prostate cancer genome-wide association studies (GWAS). Cancer Epidemiol. Biomarkers Prev. 27, 845–857 (2018).
Schumacher, F. R. et al. Association analyses of more than 140,000 men identify 63 new prostate cancer susceptibility loci. Nat. Genet. 50, 928–936 (2018).
Takata, R. et al. Genome-wide association study identifies five new susceptibility loci for prostate cancer in the Japanese population. Nat. Genet. 42, 751–754 (2010).
Akamatsu, S. et al. Common variants at 11q12, 10q26 and 3p11.2 are associated with prostate cancer susceptibility in Japanese. Nat. Genet. 44, 426–429, S421 (2012).
Xu, J. et al. Genome-wide association study in Chinese men identifies two new prostate cancer risk loci at 9q31.2 and 19q13.4. Nat. Genet. 44, 1231–1235 (2012).
Wang, M. et al. Large-scale association analysis in Asians identifies new susceptibility loci for prostate cancer. Nat. Commun. 6, 8469 (2015).
Xu, Y. et al. Family history is significantly associated with prostate cancer and its early onset in Chinese population. Prostate 79, 1762–1766 (2019).
Momozawa, Y. et al. Germline pathogenic variants in 7,636 Japanese patients with prostate cancer and 12,366 controls. J. Natl Cancer Inst. 112, 369–376 (2019).
Kim, M. et al. Clinical and pathologic characteristics of familial prostate cancer in Asian population. Prostate 80, 57–64 (2019).
Hemminki, K. & Czene, K. Age specific and attributable risks of familial prostate carcinoma from the family-cancer database. Cancer 95, 1346–1353 (2002).
Hoffmann, T. J. et al. A large multiethnic genome-wide association study of prostate cancer identifies novel risk variants and substantial ethnic differences. Cancer Discov. 5, 878–891 (2015).
Na, R. et al. Race-specific genetic risk score is more accurate than nonrace-specific genetic risk score for predicting prostate cancer and high-grade diseases. Asian J. Androl. 18, 525–529 (2016).
Maxwell, K. N., Domchek, S. M., Nathanson, K. L. & Robson, M. E. Population frequency of germline BRCA1/2 mutations. J. Clin. Oncol. 34, 4183–4185 (2016).
Pritchard, C. C. et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 375, 443–453 (2016).
Wei, Y. et al. Germline DNA repair gene mutation landscape in Chinese prostate cancer patients. Eur. Urol. 76, 280–283 (2019).
Kote-Jarai, Z. et al. BRCA2 is a moderate penetrance gene contributing to young-onset prostate cancer: implications for genetic testing in prostate cancer patients. Br. J. Cancer 105, 1230–1234 (2011).
Landrum, M. J. et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 46, D1062–D1067 (2018).
Decker, B. & Ostrander, E. A. Dysregulation of the homeobox transcription factor gene HOXB13: role in prostate cancer. Pharmgenomics Pers. Med. 7, 193–201 (2014).
Nyberg, T. et al. Homeobox B13 G84E mutation and prostate cancer risk. Eur. Urol. 75, 834–845 (2019).
Lin, X. et al. A novel germline mutation in HOXB13 is associated with prostate cancer risk in Chinese men. Prostate 73, 169–175 (2013).
VanOpstall, C. et al. MEIS-mediated suppression of human prostate cancer growth and metastasis through HOXB13-dependent regulation of proteoglycans. eLife 9, e53600 (2020).
Li, B., Huang, Q. & Wei, G.-H. The role of HOX transcription factors in cancer predisposition and progression. Cancers 11, 528 (2019).
Johng, D. et al. HOXB13 interaction with MEIS1 modifies proliferation and gene expression in prostate cancer. Prostate 79, 414–424 (2019).
Hankey, W., Chen, Z. & Wang, Q. Shaping chromatin states in prostate cancer by pioneer transcription factors. Cancer Res. 80, 2427–2436 (2020).
Zhang, J. et al. Association between germline homeobox B13 (HOXB13) G84E allele and prostate cancer susceptibility: a meta-analysis and trial sequential analysis. Oncotarget 7, 67101–67110 (2016).
Chen, Z. et al. Diverse AR-V7 cistromes in castration-resistant prostate cancer are governed by HoxB13. Proc. Natl Acad. Sci. USA 115, 6810–6815 (2018).
Brechka, H., Bhanvadia, R. R., VanOpstall, C. & Vander Griend, D. J. HOXB13 mutations and binding partners in prostate development and cancer: function, clinical significance, and future directions. Genes Dis. 4, 75–87 (2017).
Mohler, J. L. & Antonarakis, E. S. NCCN guidelines updates: management of prostate cancer. J. Natl Compr. Canc Netw. 17, 583–586 (2019).
Bernard, B. et al. Impact of ethnicity on the outcome of men with metastatic, hormone-sensitive prostate cancer. Cancer 123, 1536–1544 (2017).
Cooperberg, M. R., Hinotsu, S., Namiki, M., Carroll, P. R. & Akaza, H. Trans-Pacific variation in outcomes for men treated with primary androgen-deprivation therapy (ADT) for prostate cancer. BJU Int. 117, 102–109 (2016).
Bernard, B. et al. Impact of ethnicity on the outcome of men with metastatic, hormone-sensitive prostate cancer. Cancer 123, 1536–1544 (2017).
Levesque, E. et al. Molecular markers in key steroidogenic pathways, circulating steroid levels, and prostate cancer progression. Clin. Cancer Res. 19, 699–709 (2013).
Shiota, M. et al. Association of missense polymorphism in HSD3B1 with outcomes among men with prostate cancer treated with androgen-deprivation therapy or abiraterone. JAMA Netw. Open 2, e190115 (2019).
Hearn, J. W. D. et al. HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol. 17, 1435–1444 (2016).
Hearn, J. W. D. et al. Association of HSD3B1 genotype with response to androgen-deprivation therapy for biochemical recurrence after radiotherapy for localized prostate cancer. JAMA Oncol. 4, 558–562 (2018).
Agarwal, N. et al. Independent validation of effect of HSD3B1 genotype on response to androgen-deprivation therapy in prostate cancer. JAMA Oncol. 3, 856–857 (2017).
Almassi, N. et al. HSD3B1 and response to a nonsteroidal CYP17A1 inhibitor in castration-resistant prostate cancer. JAMA Oncol. 4, 554–557 (2018).
Shiota, M. et al. SRD5A gene polymorphism in Japanese men predicts prognosis of metastatic prostate cancer with androgen-deprivation therapy. Eur. J. Cancer 51, 1962–1969 (2015).
Shiota, M. et al. The prognostic impact of serum testosterone during androgen-deprivation therapy in patients with metastatic prostate cancer and the SRD5A2 polymorphism. Prostate Cancer Prostatic Dis. 19, 191–196 (2016).
Fujimoto, N. et al. Polymorphisms of the androgen transporting gene SLCO2B1 may influence the castration resistance of prostate cancer and the racial differences in response to androgen deprivation. Prostate Cancer Prostatic Dis. 16, 336–340 (2013).
Yang, M. et al. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 29, 2565–2573 (2011).
Wright, J. L. et al. Expression of SLCO transport genes in castration-resistant prostate cancer and impact of genetic variation in SLCO1B3 and SLCO2B1 on prostate cancer outcomes. Cancer Epidemiol. Biomarkers Prev. 20, 619–627 (2011).
Wang, X. et al. Association of SLCO2B1 genotypes with time to progression and overall survival in patients receiving androgen-deprivation therapy for prostate cancer. J. Clin. Oncol. 34, 352–359 (2016).
Shiota, M. et al. The impact of genetic polymorphism on CYP19A1 in androgen-deprivation therapy among Japanese men. Cancer Chemother. Pharmacol. 83, 933–938 (2019).
Ross, R. W. et al. Inherited variation in the androgen pathway is associated with the efficacy of androgen-deprivation therapy in men with prostate cancer. J. Clin. Oncol. 26, 842–847 (2008).
Yamada, T. et al. Genetic polymorphisms of CYP17A1 in steroidogenesis pathway are associated with risk of progression to castration-resistant prostate cancer in Japanese men receiving androgen deprivation therapy. Int. J. Clin. Oncol. 18, 711–717 (2013).
Kyriakopoulos, C. E. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J. Clin. Oncol. 36, 1080–1087 (2018).
Fizazi, K. et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 20, 686–700 (2019).
Patel, A. et al. Low-dose abiraterone in metastatic prostate cancer: is it practice changing? facts and facets. JCO Glob. Oncol. 382–386 (2020).
Parker, C. C. et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 392, 2353–2366 (2018).
Sirintrapun, S. J. & Lopez, A. M. Telemedicine in cancer care. Am. Soc. Clin. Oncol. Educ. Book 38, 540–545 (2018).
Boyle, H. J. et al. Updated recommendations of the International Society of Geriatric Oncology on prostate cancer management in older patients. Eur. J. Cancer 116, 116–136 (2019).
Roydhouse, J. K. et al. Global variation in opioid use in prostate cancer trials. JAMA Oncol. 5, e192971 (2019).
Szmulewitz, R. Z. et al. Prospective international randomized phase II study of low-dose abiraterone with food versus standard dose abiraterone in castration-resistant prostate cancer. J. Clin. Oncol. 36, 1389–1395 (2018).
Kenfield, S. A. et al. Development and application of a lifestyle score for prevention of lethal prostate cancer. J. Natl Cancer Inst. 108, djv329 (2016).
Li, X. et al. Urban-rural disparity in cancer incidence, mortality, and survivals inShanghai, China, during 2002 and 2015. Front. Oncol. 8, 579 (2018).
Remmers, S. & Roobol, M. J. Personalized strategies in population screening for prostate cancer. Int. J. Cancer 147, 2977–2987 (2020).
Piñeros, M., Znaor, A., Mery, L. & Bray, F. A global cancer surveillance framework within noncommunicable disease surveillance: making the case for population-based cancer registries. Epidemiol. Rev. 39, 161–169 (2017).
Giri, V. N. et al. Implementation of germline testing for prostate cancer: Philadelphia prostate cancer consensus conference 2019. J. Clin. Oncol. 38, 2798–2811 (2020).
Mahal, B. A. et al. Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010–2015. JAMA 321, 704–706 (2019).
Tanaka, N. et al. Trends in risk classification and primary therapy of Japanese patients with prostate cancer in Nara urological research and treatment group (NURTG) — comparison between 2004–2006, 2007–2009, and 2010–2012. BMC Cancer 17, 616 (2017).
Mitsuzuka, K. et al. Current use of active surveillance for localized prostate cancer: a nationwide survey in Japan. Int. J. Urol. 22, 754–759 (2015).
Chiu, P. K. et al. Adaptation and external validation of the European randomised study of screening for prostate cancer risk calculator for the Chinese population. Prostate Cancer Prostatic Dis. 20, 99–104 (2017).
Gronberg, H. et al. Prostate cancer diagnostics using a combination of the Stockholm3 blood test and multiparametric magnetic resonance imaging. Eur. Urol. 74, 722–728 (2018).
Chiu, P. K. et al. A multicentre evaluation of the role of the prostate health index (PHI) in regions with differing prevalence of prostate cancer: adjustment of PHI reference ranges is needed for European and Asian settings. Eur. Urol. 75, 558–561 (2019).
Gross, M. D. et al. Variation in magnetic resonance imaging-ultrasound fusion targeted biopsy outcomes in Asian American men: a multicenter study. J. Urol. 203, 530–536 (2020).
Tsukamoto, T. Editorial for National Comprehensive Cancer Network (NCCN-ACS) — Asia Consensus Statement. Jpn. J. Clin. Oncol. 49, 893–894 (2019).
Poon, D. M. C. et al. Preliminary efficacy and tolerability of chemohormonal therapy in metastatic hormone-naive prostate cancer: the first real-life experience in Asia. Asia Pac. J. Clin. Oncol. 14, 347–352 (2018).
Lee, J. L. et al. Effectiveness and safety of cabazitaxel plus prednisolone chemotherapy for metastatic castration-resistant prostatic carcinoma: data on Korean patients obtained by the cabazitaxel compassionate-use program. Cancer Chemother. Pharmacol. 74, 1005–1013 (2014).
Mahal, B. A. et al. Racial differences in genomic profiling of prostate cancer. N. Engl. J. Med. 383, 1083–1085 (2020).
Martin, A. R. et al. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat. Genet. 51, 584–591 (2019).
Strom, P. et al. Artificial intelligence for diagnosis and grading of prostate cancer in biopsies: a population-based, diagnostic study. Lancet Oncol. 21, 222–232 (2020).
Bulten, W. et al. Automated deep-learning system for Gleason grading of prostate cancer using biopsies: a diagnostic study. Lancet Oncol. 21, 233–241 (2020).
Schelb, P. et al. Classification of cancer at prostate MRI: deep learning versus clinical PI-RADS assessment. Radiology 293, 607–617 (2019).
Chen, J. et al. Genomic landscape of lung adenocarcinoma in East Asians. Nat. Genet. 52, 177–186 (2020).
Nugent, A. et al. Reporting of race in genome and exome sequencing studies of cancer: a scoping review of the literature. Genet. Med. 21, 2676–2680 (2019).
Drake, T. M., Knight, S. R., Harrison, E. M. & Soreide, K. Global inequities in precision medicine and molecular cancer research. Front. Oncol. 8, 346 (2018).
Spratt, D. E. Are we inadvertently widening the disparity gap in pursuit of precision oncology? Br. J. Cancer 119, 783–784 (2018).
Caswell-Jin, J. L. et al. Racial/ethnic differences in multiple-gene sequencing results for hereditary cancer risk. Genet. Med. 20, 234–239 (2018).
Duncan, L. et al. Analysis of polygenic risk score usage and performance in diverse human populations. Nat. Commun. 10, 3328 (2019).
Kelm, O. et al. Mapping the global cancer research funding landscape. JNCI Cancer Spectrum 3, pkz069 (2019).
Knepper, T. C. & McLeod, H. L. When will clinical trials finally reflect diversity? Nature 557, 157–159 (2018).
Egawa, S., Suyama, K., Shitara, T., Uchida, T. & Koshiba, K. Public awareness and knowledge of prostate cancer in Japan: results of a survey at short-stay examination facilities. Int. J. Urol. 5, 146–151 (1998).
Li, N. et al. Changes in clinical trials of cancer drugs in mainland China over the decade 2009–18: a systematic review. Lancet Oncol. 20, e619–e626 (2019).
Hong Kong Cancer Registry. Prostate Cancer in 2017. Hong Kong Cancer Registry https://www3.ha.org.hk/cancereg/pdf/factsheet/2017/prostate_2017.pdf (2019).
HPA China. Cancer Registry Annual Report, 2017. HPA China https://www.hpa.gov.tw/Pages/ashx/File.ashx?FilePath=~/File/Attach/12235/File_13854.pdf (2019).
UK Cancer Registry Prostate Cancer Incidence Statistics. Cancer Research UK https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer/incidence (2017).
National Cancer Center Center for Cancer Control and Information Services. Cancer Statistics in Japan. National Cancer Center Center for Cancer Control and Information Services https://ganjoho.jp/data/reg_stat/statistics/brochure/2019/cancer_statistics_2019.pdf (2020).
Saudi Health Council. National Health Information Center. Saudi Cancer Registry Cancer Incidence Report 2015. NHIC https://nhic.gov.sa/eServices/Documents/E%20SCR%20final%206%20NOV.pdf (2018).
Surveillance, Epidemiology and End Results (SEER) Program (www.seer.cancer.gov). SEER 18 2010–2016, All Races, Males by SEER Summary Stage 2000. NIH https://seer.cancer.gov/statfacts/html/prost.html (2019).
Jordan Cancer Registry. Cancer Incidence in Jordan 2012. Ministry of Health https://www.moh.gov.jo/Echobusv3.0/SystemAssets/a05a084b-3781-4979-a217-2184d5d57ede.pdf (2016).
Ministry of Public Health. Qatar Cancer Registry Qatar National Cancer Registry (QNCR) Annual Report 2015. Ministry of Public Health https://www.moph.gov.qa/_layouts/download.aspx?SourceUrl=/Admin/Lists/PublicationsAttachments/Attachments/53/QNCR-2015-English.pdf (2015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
IARC population fact sheets: https://gco.iarc.fr/today/fact-sheets-populations
Rights and permissions
About this article
Cite this article
Zhu, Y., Mo, M., Wei, Y. et al. Epidemiology and genomics of prostate cancer in Asian men. Nat Rev Urol 18, 282–301 (2021). https://doi.org/10.1038/s41585-021-00442-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00442-8
This article is cited by
-
ZNF692 promotes cell proliferation, invasion and migration of human prostate cancer cells by targeting the EMT signaling pathway
European Journal of Medical Research (2024)
-
Bridging Health Disparities: a Genomics and Transcriptomics Analysis by Race in Prostate Cancer
Journal of Racial and Ethnic Health Disparities (2024)
-
Alterations of plasma exosomal proteins and motabolies are associated with the progression of castration-resistant prostate cancer
Journal of Translational Medicine (2023)
-
Transrectal versus transperineal prostate biopsy in detection of prostate cancer: a retrospective study based on 452 patients
BMC Urology (2023)
-
Changes in the gut microbial profile during long-term androgen deprivation therapy for prostate cancer
Prostate Cancer and Prostatic Diseases (2023)