During the early peak of the COVID-19 pandemic, in March–April 2020, we assessed the impact of COVID-19 on oncology clinical trials by surveying key opinion leaders around the world and by exploring internal databases (Nat. Rev. Drug Discov. 19, 376–377; 2020). In this follow-up analysis, we have taken a targeted look at the cumulative impact of COVID-19 on oncology clinical trials around the world in the past year from April 2020 to March 2021.
Oncology trials ploughed through
On a monthly basis from April 2020 to March 2021, Cancer Research Institute (CRI) has assessed the status of trials stopped owing to COVID-19 on ClinicalTrials.gov. We found that the total number of stopped trials peaked in June 2020, whereas the number of stopped “Oncology” trials peaked in May 2020 (Fig. 1a). After these peaks, the number of stopped “Oncology” and “Other” (non-oncology) trials dropped. However, in the past 5 months since November 2020, the number of stopped “Other” trials has increased again, and COVID-19 vaccine rollout has not changed this trend. Planned and actual patient enrolment for these stopped trials peaked at 4 million, mostly owing to a large observational study.
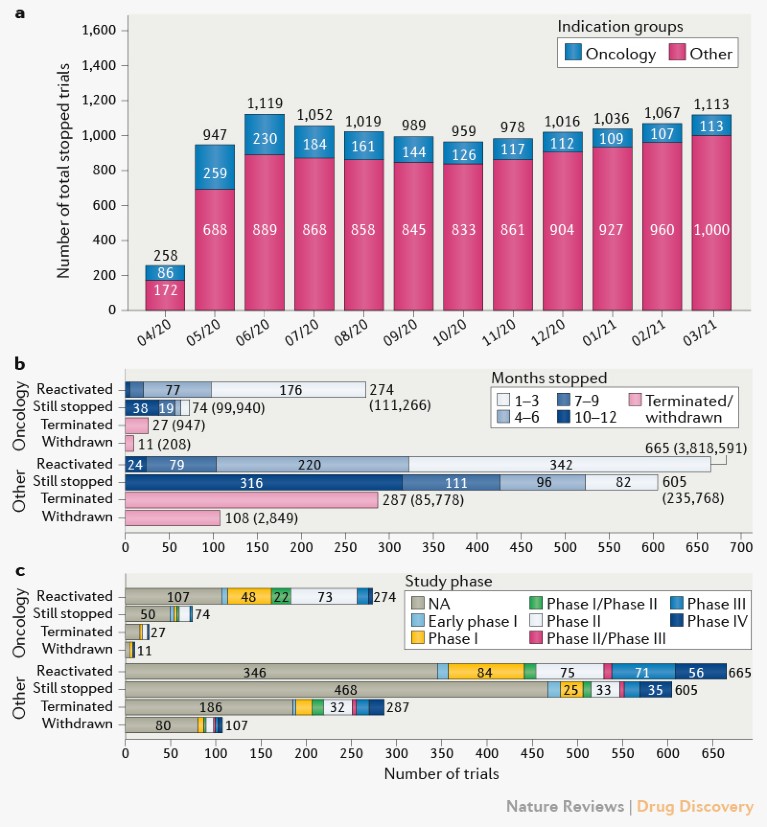
Fig. 1 | Characteristics of trials stopped owing to COVID-19. a | Trials stopped from data pull at the beginning of each month from 1 April 2020 to 1 March 2021. b | Current status of tracked trials, including duration of pause and patient enrolment in brackets. c | Status of stopped trials according to study phase. NA, not available.
To better understand how patients have been affected, we explored what proportion of halted “Oncology” and “Other” trials were interventional or observational (Supplementary Fig. 1). The number of halted oncology interventional trials peaked in May and declined faster than observational studies. “Other” interventional studies and observational studies have been stable, but stopped interventional studies have increased recently, contributing to the rise in total stopped trials observed in the past few months.
Reactivation occurred swiftly
When assessing time to reactivation or to permanent suspension, we found that 433 trials were terminated or withdrawn in the past year, 38 of which were ‘Oncology’ (Fig. 1b). The “Oncology” trials that were reactivated (274) usually did so in a matter of months, regardless of tumour type, whereas those trials that were not reactivated (74) have remained stopped for most of the year (38). Although most patient enrolment in oncology trials occurred in reactivated studies (111,266), 99,940 patients were planned to be enrolled in trials that are still stopped and nearly 1,200 patients in trials that were terminated or withdrawn. Most studies were early-phase or had non-registered phases (Fig. 1c).
Most stopped oncology trials were in solid tumour indications, with a majority in breast cancer (18%; Supplementary Fig. 2a). In the pharmacological interventional trials analysed, immuno-oncology (IO) and non-IO combinations were the dominant treatment modalities, and we observed no clear correlation between treatment modality and trial disruption (Supplementary Fig. 2b). In addition, analysis of trial sponsors revealed that 18% (374 of 2,051) of all trials stopped have an industry lead sponsor (Supplementary Fig. 3).
Conclusions
Our analyses presented here show the global impact of COVID-19 on oncology trials since April 2020. The pandemic affected all trials during the earlier waves, but the impact on oncology trials has been less severe compared with non-oncology trials. As vaccines continue to be rolled out, we expect this impact to continue to lessen.
