Abstract
Background
The current study aimed to assess the association between low maternal protein intake during pregnancy and child developmental delay at age 3 years.
Methods
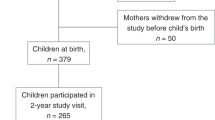
This research used data obtained from the Japan Environment and Children’s Study. In total, we analyzed 77,237 mother–child pairs. Dietary intake was assessed using the Food Frequency Questionnaire. Developmental outcomes at age 3 years were evaluated with the Japanese version of the Ages and Stages Questionnaire, Third Edition. A multivariate logistic regression analysis was performed to assess the association between maternal protein intake during pregnancy and child development delays at age 3 years.
Results
Based on the protein-to-total energy intake ratio during early pregnancy, the participants were categorized into three groups: <9.39% (>2 standard deviation below the mean), the severely low protein (SLP) group; 9.39–<13%, the low protein group; and ≥13%, the normal protein group. After adjusting for potential confounding factors, SLP intake was found to be significantly correlated with a higher risk of developmental delay according to the communication, fine motor and problem-solving skill domains.
Conclusions
SLP intake caused by inadequate diet during early pregnancy was associated with a higher risk of child developmental delay at age 3 years.
Impact
-
Animal studies have shown that maternal protein restriction during pregnancy and lactation causes abnormal brain development among offspring.
-
Birth cohort studies to date have not assessed the effects of maternal low protein exposure during pregnancy on child development.
-
Severely low protein intake during early pregnancy was associated with a higher risk of child developmental delay at age 3 years.
-
Since nutritional imbalance in early pregnancy affects not only fetal growth but also postnatal neurodevelopment, nutritional management before pregnancy is considered important.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of May 30, 2003, amendment in September 9, 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restrict the open sharing of epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
References
Walker, S. P. et al. Inequality in early childhood: risk and protective factors for early child development. Lancet 378, 1325–1338 (2011).
Suchdev, P. S. et al. Assessment of neurodevelopment, nutrition, and inflammation from fetal life to adolescence in low-resource settings. Pediatrics 139, S23–S37 (2017).
Vrijheid, M., Casas, M., Gascon, M., Valvi, D. & Nieuwenhuijsen, M. Environmental pollutants and child health: a review of recent concerns. Int. J. Hyg. Environ. Health 219, 331–342 (2016).
Barker, D. J. The origins of the developmental origins theory. J. Intern. Med. 261, 412–417 (2007).
Bianco-Miotto, T., Craig, J. M., Gasser, Y. P., van Dijk, S. J. & Ozanne, S. E. Epigenetics and DOHaD: from basics to birth and beyond. J. Dev. Orig. Health Dis. 8, 513–519 (2017).
Miguel, P. M., Pereira, L. O., Silveira, P. P. & Meaney, M. J. Early environmental influences on the development of children’s brain structure and function. Dev. Med. Child. Neurol. 61, 1127–1133 (2019).
Miyake, K. et al. DNA methylation of GFI1 as a mediator of the association between prenatal smoking exposure and ADHD symptoms at 6 years: the Hokkaido Study on Environment and Children’s Health. Clin. Epigenetics 13, 74 (2021).
Yakoob, M. Y. & Lo, C. W. Nutrition (micronutrients) in child growth and development: a systematic review on current evidence, recommendations and opportunities for further research. J. Dev. Behav. Pediatr. 38, 665–679 (2017).
Bhutta, Z. A. et al. Severe childhood malnutrition. Nat. Rev. Dis. Prim. 3, 17067 (2017).
Odhiambo, J. F., Pankey, C. L., Ghnenis, A. B. & Ford, S. P. A review of maternal nutrition during pregnancy and impact on the offspring through development: evidence from animal models of over- and undernutrition. Int. J. Environ. Res. Public Health 17, 6926 (2020).
Wu, G., Imhoff-Kunsch, B. & Girard, A. W. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr. Perinat. Epidemiol. 26, 4–26 (2012).
Herring, C. M., Bazer, F. W., Johnson, G. A. & Wu, G. Impacts of maternal dietary protein intake on fetal survival, growth, and development. Exp. Biol. Med. 243, 525–533 (2018).
Dodd, J. M. et al. Prenatal diet and child growth at 18 months. Pediatrics 142, e20180035 (2018).
Maslova, E. et al. Maternal protein intake in pregnancy and offspring metabolic health at age 9–16 y: results from a Danish cohort of gestational diabetes mellitus pregnancies and controls. Am. J. Clin. Nutr. 106, 623–636 (2017).
Barbeito-Andrés, J., Gleiser, P. M., Bernal, V., Hallgrímsson, B. & Gonzalez, P. N. Brain structural networks in mouse exposed to chronic maternal undernutrition. Neuroscience 380, 14–26 (2018).
Bautista, C. J. et al. Effects of maternal protein restriction during pregnancy and lactation on milk composition and offspring development. Br. J. Nutr. 122, 141–151 (2019).
Gould, J. M. et al. Mouse maternal protein restriction during preimplantation alone permanently alters brain neuron proportion and adult short-term memory. Proc. Natl Acad. Sci. USA 115, E7398–E7407 (2018).
Furuse, T. et al. Protein-restricted diet during pregnancy after insemination alters behavioral phenotypes of the progeny. Genes Nutr. 12, 1 (2017).
Kawamoto, T. et al. Rationale and study design of the Japan Environment and Children’s Study (JECS). BMC Public Health 14, 25 (2014).
Michikawa, T. et al. Baseline profile of participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 28, 99–104 (2018).
Yokoyama, Y. et al. Validity of short and long self-administered Food Frequency Questionnaires in ranking dietary intake in middle-aged and elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 26, 420–432 (2016).
Mezawa, H. et al. Psychometric profile of the Ages and Stages Questionnaires, Japanese translation. Pediatr. Int. 61, 1086–1095 (2019).
Black, M. M. et al. Early childhood development coming of age: science through the life course. Lancet 389, 77–90 (2017).
Donald, K. A. et al. Risk and protective factors for child development: an observational South African birth cohort. PLoS Med. 16, e1002920 (2019).
Wu, X. et al. The effect of parenting quality on child development at 36–48 months in China’s urban area: evidence from a birth cohort study. Int J. Environ. Res. Public Health 17, 8962 (2020).
Ministry of Health, Labour and Welfare. Dietary reference intakes for Japanese. https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000208954.pdf (2020).
Hennig, M. et al. Dietary protein restriction throughout intrauterine and postnatal life results in potentially beneficial myocardial tissue remodeling in the adult mouse heart. Sci. Rep. 9, 15126 (2019).
Takimoto, H., Yokoyama, T., Yoshiike, N. & Fukuoka, H. Increase in low-birth-weight infants in Japan and associated risk factors, 1980–2000. J. Obstet. Gynaecol. Res. 31, 314–322 (2005).
Gluckman, P. D., Seng, C. Y., Fukuoka, H., Beedle, A. S. & Hanson, M. A. Low birthweight and subsequent obesity in Japan. Lancet 369, 1081–1082 (2007).
Morisaki, N. et al. Optimal protein intake during pregnancy for reducing the risk of fetal growth restriction: the Japan Environment and Children’s Study. Br. J. Nutr. 120, 1432–1440 (2018).
Mathews, F., Yudkin, P. & Neil, A. Influence of maternal nutrition on outcome of pregnancy: prospective cohort study. BMJ 319, 339–343 (1999).
Lagiou, P. et al. Diet during pregnancy in relation to maternal weight gain and birth size. Eur. J. Clin. Nutr. 58, 231–237 (2004).
Chong, M. F. et al. Maternal protein intake during pregnancy is not associated with offspring birth weight in a multiethnic Asian population. J. Nutr. 145, 1303–1310 (2015).
Ji, Y. et al. Fetal and neonatal programming of postnatal growth and feed efficiency in swine. J. Anim. Sci. Biotechnol. 8, 42 (2017).
Nätt, D. et al. Perinatal malnutrition leads to sexually dimorphic behavioral responses with associated epigenetic changes in the mouse brain. Sci. Rep. 7, 11082 (2017).
Switkowski, K. M. et al. Maternal protein intake during pregnancy and linear growth in the offspring. Am. J. Clin. Nutr. 104, 1128–1136 (2016).
Geraghty, A. A. et al. Maternal protein intake during pregnancy is associated with child growth up to 5 years of age, but not through insulin-like growth factor-1: findings from the ROLO study. Br. J. Nutr. 120, 1252–1261 (2018).
Shiraishi, M., Haruna, M. & Matsuzaki, M. Effects of skipping breakfast on dietary intake and circulating and urinary nutrients during pregnancy. Asia Pac. J. Clin. Nutr. 28, 99–105 (2019).
Gressens, P. et al. Maternal protein restriction early in rat pregnancy alters brain development in the progeny. Brain Res. Dev. Brain Res. 103, 21–35 (1997).
Belluscio, L. M., Berardino, B. G., Ferroni, N. M., Ceruti, J. M. & Cánepa, E. T. Early protein malnutrition negatively impacts physical growth and neurological reflexes and evokes anxiety and depressive-like behaviors. Physiol. Behav. 129, 237–254 (2014).
Batista, T. H., Veronesi, V. B., Ribeiro, A. C. A. F., Giusti-Paiva, A. & Vilela, F. C. Protein malnutrition during pregnancy alters maternal behavior and anxiety-like behavior in offspring. Nutr. Neurosci. 20, 437–442 (2017).
Wu, G. Functional amino acids in nutrition and health. Amino Acids 45, 407–411 (2013).
Elango, R. & Ball, R. O. Protein and amino acid requirements during pregnancy. Adv. Nutr. 7, 839S–844S (2016).
Wakai, K. A review of Food Frequency Questionnaires developed and validated in Japan. J. Epidemiol. 19, 1–11 (2009).
Acknowledgements
We thank all the participants and co-operating healthcare providers for their contribution to the JECS.
Funding
This study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the above government.
Author information
Authors and Affiliations
Consortia
Contributions
K.M. conceived and designed the study, drafted the initial manuscript, and revised the manuscript. K.Mo. designed the study and reviewed and revised the manuscript. R.S., S.H., M.K., S.O., Z.Y., and the JECS group collected data and critically reviewed and revised the manuscript. Y.A., T.O., R.K., and H.Y. critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Japan Environment and Children’s Study (JECS) protocol was reviewed and approved by the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies and the Ethics Committees of all participating institutions. The JECS was conducted in accordance with the Declaration of Helsinki, and written informed consent was obtained.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miyake, K., Mochizuki, K., Kushima, M. et al. Maternal protein intake in early pregnancy and child development at age 3 years. Pediatr Res 94, 392–399 (2023). https://doi.org/10.1038/s41390-022-02435-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02435-8