Abstract
Two important maternal cardiometabolic disorders (CMDs), hypertensive disorders in pregnancy (HDP) (including pre-eclampsia) and gestational diabetes mellitus (GDM), result in a large disease burden for pregnant individuals worldwide. A global consensus has not been reached about the diagnostic criteria for HDP and GDM, making it challenging to assess differences in their disease burden between countries and areas. However, both diseases show an unevenly distributed disease burden for regions with a low income or middle income, or low-income and middle-income countries (LMICs), or regions with lower sociodemographic and human development indexes. In addition to many common clinical, demographic and behavioural risk factors, the development and clinical consequences of maternal CMDs are substantially influenced by the social determinants of health, such as systemic marginalization. Although progress has been occurring in the early screening and management of HDP and GDM, the accuracy and long-term effects of such screening and management programmes are still under investigation. In addition to pharmacological therapies and lifestyle modifications at the individual level, a multilevel approach in conjunction with multisector partnership should be adopted to tackle the public health issues and health inequity resulting from maternal CMDs. The current COVID-19 pandemic has disrupted health service delivery, with women with maternal CMDs being particularly vulnerable to this public health crisis.
Key points
-
Hypertensive disorders of pregnancy (HDP) and gestational diabetes mellitus (GDM) are common cardiometabolic complications of pregnancy.
-
HDP and GDM show an unevenly distributed disease burden (in terms of prevalence, disability-adjusted life years and/or maternal deaths) in low-income and middle-income countries and/or regions with low sociodemographic and human development indexes.
-
In addition to common clinical, demographic and behavioural risk factors, the development and clinical consequences of HDP and GDM are substantially influenced by the socioeconomic determinants of health.
-
Besides prevention and treatment at the individual level, strategies should also be made at different levels and in conjunction with multisector partnerships to improve societal and community conditions to prevent and/or manage HDP and GDM.
Similar content being viewed by others
Introduction
Two of the most common cardiometabolic disorders (CMDs) that occur during pregnancy are hypertensive disorders in pregnancy (HDP) and diabetes mellitus. HDP includes chronic hypertension, gestational hypertension and pre-eclampsia–eclampsia. Diabetes mellitus during pregnancy can be pre-existing type 1 diabetes mellitus or type 2 diabetes mellitus (T2DM), or gestational diabetes mellitus (GDM) that develops during pregnancy. This Review focuses on HDP and GDM. HDP and GDM share many common risk factors and similarities in their pathophysiology, including oxidative stress, inflammation and vascular endothelial dysfunction1; these two maternal conditions result in a large disease burden for both pregnant individuals and their offspring. Despite decreasing prevalence after years of interventions, HDP remain a leading cause of maternal mortality and morbidity globally, especially in low-income and middle-income countries (LMICs)2,3,4. The prevalence of GDM has increased dramatically over the past two decades by more than 30% in numerous countries5,6,7. These two maternal CMDs are related to substantial short-term and long-term adverse health outcomes for pregnant individuals and their offspring. Individuals with HDP or impaired glucose metabolism during pregnancy experience greater maternal mortality and morbidity rates than people with uncomplicated pregnancies. Furthermore, pregnant people with HDP or impaired glucose metabolism have an increased risk of future CMDs and premature death later in life8,9,10. Negative influences of HDP and hyperglycaemia during pregnancy on fetuses and neonates include, but are not limited to, intrauterine growth restriction (IUGR) and macrosomia, preterm birth, low birthweight and adverse outcomes later in life11. Notably, the burden of premature deaths from complications of CMDs in pregnancy and associated cardiovascular disease (CVD) later in life falls disproportionately upon LMICs. Several socioenvironmental factors, including poverty, air pollution, educational and sociocultural barriers, and limitations in health-care access and infrastructure12, are responsible for such inequities in disease burden.
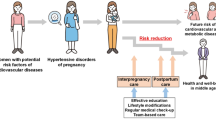
This Review discusses the global disease burden and risk factors for HDP and GDM, highlighting the differences between high-income countries (HICs) and LMICs. In addition, we provide policy recommendations regarding public health interventions that can be contextualized and implemented either worldwide or regionally to help reduce the mortality and morbidity related to these maternal CMDs in an efficient and cost-effective manner. We note that, unless otherwise specified, the terms women and men refer to ciswomen and cismen.
Diagnostic criteria
HDP
Comprising chronic hypertension, gestational hypertension and pre-eclampsia–eclampsia, the precise definition and classification of HDP is evolving over time, especially for pre-eclampsia. Pre-eclampsia is not a single disorder but a variety of pathophysiological pathways that converge on a common syndromic end point, of high blood pressure occurring with proteinuria after 20 weeks of pregnancy13. In the past 10 years, the definition of pre-eclampsia has been extended to include individuals without proteinuria but with evidence of maternal end-organ or uteroplacental dysfunction14. Two of the broad definitions adopted by most clinical practice guidelines and authorities are those of the International Society for the Study of Hypertension in Pregnancy15 and the American College of Obstetricians and Gynecologists16 (Supplementary Table 1). The application of these broad definitions of pre-eclampsia means patients once diagnosed with gestational hypertension or chronic hypertension were recategorized as pre-eclampsia or chronic hypertension with superimposed pre-eclampsia, respectively17,18,19,20,21. This diagnostic shift will influence clinical management (for example, increased hospital admission and induction of labour)17. Although the debate is still ongoing on these new classification systems, studies published in 2021 revealed that a broad definition of pre-eclampsia could better identify women and babies at risk of adverse outcomes22,23.
GDM
The diagnostic criteria for GDM have also evolved (Supplementary Table 2) These criteria are usually based on glucose thresholds for oral glucose tolerance tests. Currently, the screening and diagnostic approaches for GDM are under debate24,25,26,27,28,29,30,31,32 given differences in the focus of different guidelines. For example, the ability of the diagnostic criteria to predict the risk of adverse maternal and neonatal outcomes33,34,35 versus the maternal risk of developing T2DM in the future36,37,38. Adopting broad definitions of GDM might result in a considerable increase in the prevalence and incidence of GDM26,39, with potentially increased health-care costs40 and psychological stress in women and their families41. However, many researchers consider GDM treatment highly cost-effective when the benefits of future maternal T2DM and childhood obesity risk reduction are taken into account32,42,43,44,45. Therefore, a majority of health authorities, such as the WHO and the American Diabetes Association, have come to support the broad criteria, such as the criteria of the International Association of the Diabetes and Pregnancy Study Groups (IADPSG)26,39.
Disease burden
HDP
HDP are among the leading causes of maternal and fetal morbidity and mortality worldwide3, responsible for an estimated 14% of maternal deaths globally. Despite a much lower maternal mortality in HICs than in LMICs, HDP remains one of the most common causes of maternal death worldwide2,3,4. The proportion of maternal deaths from HDP was 2.8% in the UK and Ireland (2011–2013), whereas maternal mortality related to HDP ranged between 0.08 and 0.42 per 100,000 pregnancies between 2009 and 2015 (ref.46). The proportion of maternal deaths attributable to HDP is 7.4% in the USA, accounting for an estimated one-fifth of antenatal admissions and two-thirds of referrals to daytime assessment units4. In France, HDP account for one-quarter of obstetric admissions to intensive care units4. In contrast, in LMICs, 10–15% of direct maternal deaths are associated with HDP3,4. Therefore, epidemiological surveillance of HDP is crucial for perinatal health care all over the world.
To illustrate the global prevalence of HDP, we extracted data on prevalence, death and disability-adjusted life years (DALYs) from the Institute for Health Metrics and Evaluation (IHME) Global Burden of Disease (GBD) 2019 report47. The GBD report estimates health loss due to 369 diseases and injuries for more than 200 countries and territories all over the world. A critical resource for informed policy making, the GBD report is aimed at improving health systems and eliminating health inequities48. Globally, the prevalence of HDP is 116.4 per 100,000 women of childbearing age. At the regional level, Africa had the highest prevalence of HDP, with a mean prevalence of 334.9 per 100,000 women of childbearing age, followed by Southeast Asia and the Middle East, with mean prevalences of 136.8 and 121.4 per 100,000 women of childbearing age, respectively. Conversely, the Western Pacific region had the lowest prevalence of HDP at 16.4 per 100,000 women of childbearing age (Fig. 1). A great disparity exists between HICs and LMICs regarding the disease burden of HDP (Table 1).
The mean prevalence of hypertensive disorders in pregnancy (HDP) shows a state of inequity among different regions worldwide. Africa has the highest mean prevalence of HDP, which is far higher than in other regions. It is followed by South East Asia and Eastern Mediterranean, which have a mean prevalence of HDP of over 0.1% among women of childbearing age. The Western Pacific has the lowest mean prevalence of HDP. Data were originally presented in ref.48.
To further illustrate potential differences in disease burden of HDP at the country level, we stratified the disease burden (prevalence, DALYs and death) of HDP by country and sociodemographic index (SDI) and human development index (HDI), respectively. Countries with lower SDI and HDI generally had a greater disease burden of HDP than those with higher SDI and HDI, demonstrated by a higher prevalence, DALYs and death attributable to HDP (Figs. 2–4). These data are consistent with those of other studies49. The WHO’s estimate of the incidence of HDP in developing countries is 2.8% of live births, compared to an incidence of 0.4% of live births in developed countries3. A 2021 study investigating the epidemiological trends of HDP by using the GBD data showed that the death and incidence rates of HDP are decreasing in most countries and all regions except for those with low SDI and HDI49.
Each dot indicates the prevalence of hypertensive disorders in pregnancy (HDP) in a particular country or region in relation to the sociodemographic index (SDI; part a) and human development index (HDI; part b) in 2019. The size of each dot reflects the total population of the corresponding country or region. Notable countries and regions are labelled. Countries and regions with the lowest SDI and/or HDI have the highest prevalence of HDP, including Somalia, Nigeria, Chad, Burkina Faso, Mali and Burundi. Data were originally presented in ref.48.
Each dot indicates the disability-adjusted life years (DALYs) due to hypertensive disorders of pregnancy (HDP) in a particular country or region in relation to the sociodemographic index (SDI; part a) and human development index (HDI; part b) in 2019. The size of each dot reflects the total population of the corresponding country or region. Notable countries and regions are labelled. Countries and regions with low SDI and/or HDI have high DALYs attributable to HDP, including Pakistan, Sierra Leone, Rwanda, Haiti and Guinea. Data were originally presented in ref.48.
Each dot indicates the maternal deaths attributable to hypertensive disorders in pregnancy (HDP) in a particular country or region in relation to the sociodemographic index (SDI; part a) and human development index (HDI; part b) in 2019. The size of each dot reflects the total population of the corresponding country or region. Notable countries and regions are labelled. Countries and regions with low SDI and/or HDI have high numbers of maternal deaths attributable to HDP, including Pakistan, Sierra Leone, Rwanda and Haiti. Data were originally presented in ref.48.
GDM
The global prevalence of GDM has also steadily increased in the past four decades. Depending on the diagnostic criteria used, 9–25% of pregnancies are affected by GDM50. According to a global observational study, the prevalence of GDM ranged between 9% and 26% in 15 centres51. The rapid global increase in GDM occurring within the past few decades has created an emerging epidemic in both HICs and LMICs52. We extracted data related to pre-existing diabetes mellitus in pregnancy and GDM from the International Diabetes Federation report (10th edition)53 (Fig. 5). Southeast Asia had the highest prevalence of GDM, with a median estimate of 25.9%, followed by the North American and Caribbean regions (median prevalence 20.7%). With a median prevalence of 14.0%, the Western Pacific had the lowest prevalence of GDM. These data are comparable to those of previous studies7,54. A great disparity exists between HICs and LMICs regarding the disease burden of GDM (Table 2). However, given different diagnostic criteria for GDM used by different countries, strict cross-country/region comparisons are difficult to interpret (Fig. 6).
The mean prevalence of gestational diabetes mellitus (GDM) (the percentage of all pregnant women with GDM) shows a state of inequity among different regions worldwide. South East Asia has the highest mean prevalence of GDM, followed by North America and the Caribbean. Africa has the lowest mean prevalence of GDM. Data were originally presented in ref.53.
Prevalence of gestational diabetes mellitus (GDM) in pregnant women by country according to different diagnostic criteria: American Diabetes Association (ADA), International Association of the Diabetes and Pregnancy Study Groups (IADPSG), WHO, European Association for the Study of Diabetes (EASD), and other International Classification of Diseases codes and local guidelines or criteria (Others). SAR, Special Administrative Region. Data were originally presented in ref.53.
Risk factors
Common risk factors
HDP and GDM share several risk factors, including advanced maternal age55,56,57,58,59,60,61,62,63,64, overweight or obesity56,63,65,66,67,68,69,70, nutrition (such as reduced calcium and vitamin D3 intake71,72,73,74,75,76,77) and dietary patterns before and/or during pregnancy. For instance, a low intake of fruit, green leafy vegetables, poultry and fish, and high consumption of the Western dietary pattern (characterized by a high intake of red meat, processed meat, refined grain products, high-fat and/or high-sugar processed food) might be associated with an elevated risk of HDP and GDM78,79,80,81,82,83,84,85,86,87 (Fig. 7).
Common risk factors for hypertensive disorders in pregnancy (HDP) are shown on the left, and common risk factors for gestational diabetes mellitus (GDM) are shown on the right. The overlapping area in the centre shows the risk factors shared by both HDP and GDM. PCOS, polycystic ovary syndrome; T2DM, type 2 diabetes mellitus.
Some obstetric complications and situations, including primiparity56,58,88,89,90,91, multifetal pregnancy56,57,58,88,91 and history of GDM56,91,92, are related to the development of HDP16,56,65,66,88,90,91,93,94,95,96,97. Other risk factors for HDP include a previous history of HDP93,98,99, a family history of HDP93 and pre-existing diseases, such as chronic hypertension, pregestational diabetes mellitus, thrombophilia, systemic lupus erythematosus, antiphospholipid antibody syndrome, kidney disease and obstructive sleep apnoea16,93,94,97. Smoking has been revealed to be a potential protective factor for HDP100, but the evidence of the association between smoking during pregnancy and HDP remains controversial101,102.
In terms of GDM, a previous history of GDM and a family history of diabetes mellitus might increase the risk of developing GDM in a current pregnancy63. Other potential risk factors include carrying a male fetus103,104,105,106, parity7 and polycystic ovarian syndrome107, although some evidence is not very consistent108. By contrast, physical activity before and during pregnancy was reported to be associated with a decreased risk of GDM80,109,110,111.
Race, ethnicity, socioeconomic and environmental factors
Debate is ongoing on the role of race and/or ethnicity in the development of maternal CMDs. Certain ethnic and racial groups have been widely reported to have a disproportionately increased disease burden of maternal CMDs. For example, African American women and Filipino women have an increased risk of developing HDP112,113,114. Higher incidence rates of HDP have also been found in Māori, Indigenous Australian, American Indian and Alaskan Native populations94,115,116,117, whereas the risk of HDP in Pacific Islander populations is still controversial118,119. Racial and ethnic groups with an increased risk of developing GDM include Indigenous Australian, Pacific Islander, South or East Asian, Middle Eastern, Hispanic and African populations6,120,121,122,123,124,125,126,127,128. However, whether race and/or ethnicity are independent, genetically determined risk factors for maternal CMDs is controversial. Researchers have found that individuals of African Caribbean origin have a higher risk of developing HDP than white individuals, even after adjusting for markers of social deprivation129. Some biomarkers of disease risk have also been found to vary according to racial origin. For instance, circulating levels of placental growth factor (PlGF) in Black women and South and East Asian women are higher than in white women130. Some genetic variants have been reported to be associated with HDP in women with GDM1, including the MIR146A rs2910164CC131, HNF1A p.I27LTT132 and ACE I/D polymorphism DD133 genotypes. Of note, race can be considered as a social construct rather than a biological construct114,134, as race is a socially derived label that can either be self-reported or assigned and might not justify any biological or genetic differences between populations135,136. For example, many population studies found more genetic variations within racial groups than among them137,138.
The social determinants of health (SDOH)139 are defined as the non-medical conditions in which people are born, grow, work, live and age, as well as the broader set of forces and systems that shape daily life conditions. According to Healthy People 2030 (ref.140), SDOH can be grouped into five domains: economic stability, education access and quality, health care and quality, neighbourhood and built environment, and social and community context. These factors influence health outcomes and therefore have an important influence on health inequities. The association of maternal social adversities and unfavourable pregnancy outcomes with offspring health has been widely studied and established. For example, social stress, malnutrition during pregnancy and environmental toxins have been proposed as three SDOH factors that might affect placental health141. In accordance with epigenetic drivers and genetic predisposition, maternal social adversities result in insidious placental changes and/or malfunction and could lead to adverse outcomes during pregnancy and beyond.
Certain racial and/or ethnic groups have an increased prevalence of maternal CMDs, but, as mentioned above, this phenomenon cannot be fully explained by genetic background. In the Generation R study142,143 (a large population-based California cohort of singleton births)144, Black women had an increased risk of HDP compared with white women. Higher socioeconomic status (SES), whether indicated by education or insurance status, further reduced the risk of HDP in white pregnant individuals, which in turn indirectly predicted longer gestation length. High SES is not as health-protective for Black individuals, which might be explained by structural and cultural forms of racism they experience despite their SES144. HDP was found to mediate the association between racial residential segregation and low birthweight among Black women in New York City, USA145. Furthermore, racial residential segregation was associated with higher odds of HDP in areas with higher neighbourhood poverty rates than in those with lower rates146, which had implications for racial disparities in adverse pregnancy outcomes and CVD later in life. The stress of systemic racial disparities, such as poverty, living in racially segregated neighbourhoods, a lack of access to health-care services and experience of discrimination, can all negatively affect the health of women of certain ethnic groups, such as non-Hispanic Black women147.
Evidence also supports associations between SDOH and diabetes mellitus-related outcomes148. Inequities in SDOH notably impact disparities in diabetes mellitus risk, diagnosis and outcomes149,150, and diabetes mellitus during pregnancy is no exception151. Disparities in SDOH can lead to different maternal and neonatal outcomes in pregnant women with diabetes mellitus. SES factors, such as education, occupation and household income, have been reported to be associated with GDM152, but study findings are inconsistent6,143,153,154,155,156,157. Some environmental factors, such as passive smoking156 and exposure to persistent organic pollutants (POP) or endocrine disruptors158,159, might contribute to an increased risk of developing GDM. A prospective study demonstrated a modest association between depressive symptoms early in pregnancy and an increased risk of incident GDM, particularly in women without obesity and women with persistent depressive symptoms throughout the first two trimesters of pregnancy160.
The association between air pollution and maternal CMDs is a frequent topic of investigation. Several studies have shown relationships between perinatal exposure to particulate matter ≤2.5 µm in size (PM2.5) and placental oxidative stress, DNA damage, inflammation, hypercoagulation and thrombosis161,162,163,164,165,166, all of which are considered factors associated with the occurrence of maternal CMDs. A systematic review and meta-analysis included 11 studies and found that PM2.5, nitrogen oxides and SO2 exposure increased the risk of GDM167. Another study investigated the association between indoor air pollution and pre-eclampsia and indicated a twofold greater risk of reporting pre-eclampsia symptoms in women living in households using biomass and solid fuels than those living in households using clean fuels168. A systematic review was conducted on environmental contaminants and pre-eclampsia, which included studies examining POPs (six studies), drinking water contaminants (one study), atmospheric pollutants (11 studies), metals and metalloids (six studies), and other environmental contaminants (four studies)169. Although definitive conclusions could not be drawn on most chemicals due to the insufficiency of investigations, nitrogen dioxide, PM2.5 and traffic exposure were suggested to be associated with pre-eclampsia. Similarly, the impact of environmental chemicals (for example, bisphenol A, phthalates and toxic metals) on the development of GDM is not consistent among studies170. In general, the current evidence is highly heterogeneous. Moreover, humans are exposed to complex mixtures of various environmental contaminants, making it difficult to isolate the effect of a single chemical from those of other unknown or unmeasured co-exposures. Studies large enough to give rise to an adequate number of maternal CMD cases and equipped with robust methodology are needed to identify or confirm the relationship of maternal CMDs and environmental pollutants, to inform policy making or to develop behavioural interventions.
Clinical consequences
Maternal CMDs, such as HDP and GDM, can lead to various obstetric complications such as preterm birth, placental abruption and postpartum haemorrhage33,171. Furthermore, they can have negative perinatal outcomes for both the mother and the fetus or neonate, such as maternal end-organ injuries, maternal death, IUGR, large for gestational age, shoulder dystocia, hypoglycaemia, birth asphyxia, respiratory distress syndrome33,171,172, congenital malformations in neonates173,174, stillbirth and neonatal death. Importantly, these complications might generate long-term health problems for these mothers and their offspring.
Women with a history of HDP are more likely to have recurrent HDP in subsequent pregnancies, and this risk increases with decreasing gestational age at delivery in the index pregnancy175. HDP is also independently associated with a higher risk of T2DM in the future176. Moreover, women with a history of HDP are at a higher risk of developing hypertension, CVD and CVD-related morbidity and mortality than women with uncomplicated pregnancies97,177,178,179,180,181,182,183,184,185,186,187,188 (Supplementary Table 3). GDM had similar effects on the risk of women developing future CVDs189,190 (Supplementary Table 4), independent of obesity and at a fairly young age191,192,193,194. Women with GDM have a much higher risk of developing impaired glucose tolerance and T2DM in later life than women with uncomplicated pregnancies7,195,196,197,198,199,200,201 (Supplementary Table 5). This risk is especially high for individuals with a high severity or postpartum continuation of glucose intolerance and high BMI199,200,201. The diagnosis of GDM early in pregnancy, such as during the first half of pregnancy, might increase the risk of developing diabetes mellitus later in life202. However, the evidence is inconsistent203.
The theory of developmental origins of health and disease underlines the role of both prenatal and postnatal environments in shaping developmental trajectories on long-term health204. Available evidence has indicated, in addition to affecting the long-term health of mothers, that maternal CMDs could exert harmful health burdens on their offspring later in life. Neonates exposed to HDP might have higher blood pressure when entering adolescence than neonates from healthy pregnancies205,206,207,208. Evidence also exists of a link between HDP and later-life CVD and cerebrovascular disease in pregnant individuals, although it is unclear whether HDP impair the maternal CVD system and result in future CVD in these pregnant individuals, or whether they share common risk factors209. Maternal diabetes mellitus, regardless of the type (that is, pre-existing type 1 diabetes mellitus or T2DM, or GDM), has long-term effects on the risk of diabetes mellitus and obesity in offspring172,210,211,212,213,214,215,216.
In the past decade, long-term neurological and psychiatric outcomes in neonates born to mothers with maternal CMDs have received much attention. The offspring of women with HDP are reported to be at a greater risk of developing cognitive and psychiatric disorders, such as autism spectrum disorder (ASD), attention-deficit–hyperactivity disorder (ADHD)217,218,219 or epilepsy during their later life220. Evidence is also emerging of the relationship between GDM and neuropsychiatric conditions in children. A 2021 systematic review found an increased risk of developing ASD but not ADHD in offspring when exposed to GDM221. Of note, the role of confounders, mediators and effect modifiers (for example, gestational age at birth, birthweight and SES) were not explored in many of these studies, making it difficult to interpret the current findings.
Prevention and treatment
Given the large disease burden following maternal CMDs such as HDP and GDM, for decades, researchers have explored treatments that can not only solve short-term problems but also prevent or improve long-term health outcomes for mothers with maternal CMDs and their offspring.
Treatment of maternal CMDs in the clinical setting
Currently, several treatment strategies for HDP are applied in the clinical setting, such as calcium, vitamin D or folic acid supplementation, or treatment with aspirin or anti-platelet agents (Supplementary Table 6). Other novel approaches have been investigated in clinical or preclinical studies for their benefits in preventing or treating HDP, including metformin222,223,224,225,226,227, pravastatin228,229,230,231,232,233, proton pump inhibitors234,235,236, sulfasalazine, antioxidants (for example, melatonin, MitoQ, polyphenols, and vitamins C and E)237, sildenafil citrate238,239 and biological therapies (such as monoclonal antibodies)240,241. Placenta-specific drug delivery systems, such as the application of nanoparticles, have also been developed to prevent off-target effects from the systemic administration of certain medications. Several animal studies (most commonly using mice or rats) in this area have been performed; for example, using polyamidoamine to carry short-interfering RNA to silence the gene encoding soluble fms-like tyrosine kinase 1 (FLT1) and to decrease secretion of the gene product242. Furthermore, synthetic placental chondroitin sulfate A-binding peptide has been used to target trophoblasts243. Preclinical studies of monoclonal antibodies targeting tumour necrosis factor, PlGF and complement are underway as well237. Of note, current studies on placenta-targeted treatments, which might enable safe and efficient delivery of therapeutic drugs to improve pregnancy outcomes, mainly focus on short-term health outcomes (for example, fetal growth or birthweight)244. The choice of the most appropriate time point and the dosage and frequency to administer therapeutic interventions and the assessment of the long-term effects of these treatments on improving later-life health outcomes in offspring remain challenges in this area.
Treatments for GDM aim to achieve satisfactory glycaemic control to improve the short-term and long-term health of both mothers and babies. A wide variety of management strategies, from lifestyle interventions (such as diet and exercise) to pharmacological medications (such as metformin and insulin), have been assessed for their effectiveness and safety. A package of care (a combination of treatments starting with dietary modifications and/or exercise and/or pharmacological treatments) is effective in reducing the risk of most adverse perinatal outcomes of GDM, but the evidence is of low quality245. An overview of Cochrane reviews also found there is insufficient high-quality evidence about the effects of various interventions in GDM246.
Prevention and long-term management of maternal CMDs
To date, very limited evidence exists regarding effective approaches for preventing the development of maternal CMDs and their negative health outcomes. An overview of Cochrane reviews was conducted on the effects of various interventions (diet, exercise, diet and exercise combined, dietary supplements, pharmaceutical management such as metformin, and the management of other health issues) for preventing GDM247. The researchers found effects only for combined diet and exercise interventions during pregnancy and supplementation with myo-inositol, vitamin D and treatment with metformin, but the evidence was of low to moderate quality. In another Cochrane review248, the average risk reduction from lifestyle interventions on HDP was 0.70 (95% CI 0.40–1.22; four trials, 2,796 women; I2 = 79%; low-quality evidence). The long-term impact of lifestyle interventions on neonates, such as diabetes mellitus and adiposity in adulthood and neurosensory disability in later childhood, is rarely reported.
Lifestyle interventions include a wide variety of components (for example, education, diet, exercise and self-monitoring of blood levels of glucose). Currently, no clear evidence is available of the effectiveness of lifestyle interventions in preventing the development of HDP. Probiotic-related interventions that target the microbiota might be able to improve glycaemic control in women with GDM249. However, many aspects of probiotic intervention remain unclear, including the underlying mechanism, type, dose and duration of probiotics that are safe for administration during pregnancy, and whether the offspring of mothers with GDM could have long-term benefits from probiotic interventions249,250.
Contrary to the great achievements that have been made by health professionals in understanding and managing CMDs in pregnancy, patient education lags greatly251,252. Self-management involving lifestyle modification and regular glucose monitoring is crucial for the management of pre-existing diabetes mellitus in pregnancy and GDM253. Improved understanding of GDM, nutrition and self-management principles may result in improved glucose levels and a reduction in the number of individuals requiring insulin treatment254,255,256,257.
Interventions for maternal CMDs from the public health perspective
CMDs in pregnancy have already become a complex public health issue since they result in an increased disease burden and generate a profound impact on health worldwide. To tackle this public health problem and reduce disparity, a multilevel approach258 should be adopted. In addition to prevention and treatment at the individual level by addressing individual lifestyle and behavioural factors that influence health, strategies should be made at different levels and in conjunction with multisector partnerships to improve societal and community conditions by addressing the SODH.
Obesity56,65,66,67 and certain dietary patterns such as the Western dietary pattern85 are risk factors for hypertension and hyperglycaemia in pregnancy. Calcium insufficiency71, and extremely young maternal age55,56,58 are also recognized risk factors for HDP. Policies and measures to ensure food security, to help with dietary diversity and to delay marriage or first pregnancy (for example, until after 20 years old) might therefore help reduce the disease burden arising from CMDs in pregnancy. Although study findings have been inconsistent6,143,153,154,155,156,157, poverty and poor living conditions might be associated with the development of CMDs in pregnancy153. Given that environmental factors such as indoor air pollution168, passive smoking156, POPs, and endocrine disruptors158,159 might contribute to an increased risk of developing either HDP or GDM in pregnancy, legislation, policies, interventions and advocacy activities for smoking cessation and pollution control might lead to a decreased incidence of these two disorders.
At the community and healthcare facility level, early detection and proper management of CMDs during pregnancy, tailored to various settings and populations are crucial. In resource-limited areas where multiple clinic visits might not be possible, a point-of-care approach could be adopted. In addition, rural health workers should receive enhanced training to improve community-level detection and management of CMDs in pregnancy. For example, the community-level interventions for pre-eclampsia (CLIP) trials in Mozambique, Pakistan and India involved community engagement and task sharing with community health workers for triage and initial treatment of HDP in the local pregnant population. The findings from the CLIP trials suggest that community-level interventions for women with HDP can be successfully completed by community health workers, but their numbers must be adequate to provide at least eight antenatal care contacts to reduce adverse outcomes259. Among women who received eight or more CLIP contacts (four in Pakistan), the probability of health system and family cost-effectiveness was ≥80%260. However, the CLIP study did not generate a statistically significant reduction in all-cause maternal and perinatal mortality or morbidity. This finding suggests that a focus only on community-level intervention without facility enhancement is inadequate to improve maternal and neonatal outcomes259.
Several successful community-based GDM programmes have been conducted, some of which targeted specific at-risk groups and addressed health inequities. For instance, a programme with diabetes mellitus-specific infrastructure including certified diabetes educator visits and diabetes group visits was carried out in a high-risk population of pregnant Latino women, and demonstrated improved glycaemic control261. Successful community-based diabetes mellitus programmes can serve as models for programmes targeting diabetes mellitus in pregnancy. Consulting and mobilizing effective and existing community and village leadership and infrastructure enables the delivery of community-based programmes. Several key elements must be in place to implement these community-based programmes: first, a data collection and tracking system to capture and record the information from all sources of patient care, interactions and outcomes that the programme aims to achieve; second, a well-structured staff team; third, a training schedule for all who will be participating in programme delivery; fourth, health system integration via a shared electronic medical system; fifth, the identification of additional local resources that are easily available to patients to assist them in achieving their clinical and behavioural goals; and finally, ongoing communication among all parties. Furthermore, low-cost devices (such as an alert device or urinalysis device) and mobile health technologies can also have important roles in improving the outcomes of CMDs during pregnancy in remote and resource-limited areas.
The current COVID-19 pandemic has greatly disrupted health service delivery due to lockdown policies, overwhelmed health-care systems and exhausted health-care providers among other effects, such as exacerbation of poverty. Maternal and neonatal health services are no exception, particularly in resource-limited countries262. A prospective observational study was conducted in Nepal263, which collected participant-level data for pregnant women enrolled in two other studies during the COVID-19 pandemic. The study found that institutional childbirth was reduced by more than half during lockdowns, along with an increase in the institutional stillbirth rate and neonatal mortality and decreased quality of care. Women with CMDs, such as HDP and GDM, in pregnancy are at a higher risk of adverse perinatal outcomes than those with uncomplicated pregnancies and require more intensive antenatal care. The COVID-19 pandemic might therefore generate large negative impacts on this population.
In terms of maternal CMDs, it was found that COVID-19 and pre-eclampsia impact perinatal outcomes (such as preterm birth, severe perinatal morbidity and mortality) in an additive fashion264. T2DM is one of the characteristics of patients who are at high risk of severe COVID-19 or death265,266,267,268. However, there are only a limited number of studies in women with GDM who are also infected with SARS-CoV-2. In the context of this and future pandemics, especially when the lockdown of general services occurs, it can be challenging for pregnant women to receive an oral glucose tolerance test and for those with hyperglycaemia in pregnancy to receive relevant health service visits for diabetes education, glucose monitoring review, fetal ultrasonography and eye testing269. All these factors might lead to a decreased quality of care and worsen outcomes for patients with pre-existing diabetes mellitus in pregnancy and GDM. Consequently, women with or at high risk of CMDs in pregnancy should receive special attention and preventive care during future emergencies and health service disruptions.
Conclusions
Two major maternal CMDs, HDP and GDM, are related to substantial short-term and long-term adverse health outcomes for women and their offspring. HDP and GDM have resulted in a large disease burden globally, especially among LMICs. Much progress has been made in understanding the disease burden, risk factors and clinical consequences of HDP and GDM. However, further research is needed to study the underlying pathophysiology, to develop accurate and reliable early screening and diagnostic tools, and to explore novel, effective and safe treatment strategies at the population level. Sensitive and reliable diagnostic criteria or classification lay a solid ground for epidemiology and clinical research. In addition to clinical management, a multilevel public health strategy is required to ameliorate the disease burden and to address the health inequities related to maternal CMDs.
References
Yang, Y. & Wu, N. Gestational diabetes mellitus and preeclampsia: correlation and influencing factors. Front. Cardiovasc. Med. 9, 831297 (2022).
Steegers, E. A., von Dadelszen, P., Duvekot, J. J. & Pijnenborg, R. Pre-eclampsia. Lancet 376, 631–644 (2010).
Say, L. et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob. Health 2, e323–e333 (2014).
Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 33, 130–137 (2009).
Ferrara, A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 30, S141–S146 (2007).
Anna, V., van der Ploeg, H. P., Cheung, N. W., Huxley, R. R. & Bauman, A. E. Sociodemographic correlates of the increasing trend in prevalence of gestational diabetes mellitus in a large population of women between 1995 and 2005. Diabetes Care 31, 2288–2293 (2008).
Zhu, Y. & Zhang, C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr. Diab. Rep. 16, 7 (2016).
Rich-Edwards, J. W., Fraser, A., Lawlor, D. A. & Catov, J. M. Pregnancy characteristics and women’s future cardiovascular health: an underused opportunity to improve women’s health? Epidemiol. Rev. 36, 57–70 (2014).
Hauspurg, A., Ying, W., Hubel, C. A., Michos, E. D. & Ouyang, P. Adverse pregnancy outcomes and future maternal cardiovascular disease. Clin. Cardiol. 41, 239–246 (2018).
Heida, K. Y. et al. Cardiovascular risk management after reproductive and pregnancy-related disorders: a Dutch multidisciplinary evidence-based guideline. Eur. J. Prev. Cardiol. 23, 1863–1879 (2016).
Morken, N. H., Halland, F., DeRoo, L. A., Wilcox, A. J. & Skjaerven, R. Offspring birthweight by gestational age and parental cardiovascular mortality: a population-based cohort study. BJOG 125, 336–341 (2018).
Nagraj, S. et al. Cardiometabolic risk factors in pregnancy and implications for long-term health: identifying the research priorities for low-resource settings. Front. Cardiovasc. Med. 7, 40 (2020).
Roberts, J. M. & Bell, M. J. If we know so much about preeclampsia, why haven’t we cured the disease? J. Reprod. Immunol. 99, 1–9 (2013).
Roberts, J. M., Rich-Edwards, J. W., McElrath, T. F., Garmire, L. & Myatt, L. Subtypes of preeclampsia: recognition and determining clinical usefulness. Hypertension 77, 1430–1441 (2021).
Brown, M. A. et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 72, 24–43 (2018).
The American College of Obstetricians and Gynecologists. Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet. Gynecol. 135, e237–e260 (2020).
Bouter, A. R. & Duvekot, J. J. Evaluation of the clinical impact of the revised ISSHP and ACOG definitions on preeclampsia. Pregnancy Hypertens. 19, 206–211 (2020).
Khan, N. et al. Impact of new definitions of pre-eclampsia on incidence and performance of first-trimester screening. Ultrasound Obstet. Gynecol. 55, 50–57 (2020).
Kallela, J. et al. The diagnosis of pre-eclampsia using two revised classifications in the Finnish Pre-eclampsia Consortium (FINNPEC) cohort. BMC Pregnancy Childbirth 16, 221–221 (2016).
Reddy, M. et al. The impact of the definition of preeclampsia on disease diagnosis and outcomes: a retrospective cohort study. Am. J. Obstet. Gynecol. 224, e211–e217 (2021).
Saleh, L., Danser, J. A. H., Van Den Meiracker, A. H. & Visser, W. The prevalence of hypertensive disorders according to the old and new criteria of ISSHP and ACOG: risk factors, prediction of preeclampsia. Pregnancy Hypertens. 6, 229 (2016).
Lai, J., Syngelaki, A., Nicolaides, K. H., von Dadelszen, P. & Magee, L. A. Impact of new definitions of preeclampsia at term on identification of adverse maternal and perinatal outcomes. Am. J. Obstet. Gynecol. 224, e511–e518 (2021).
Magee, L. A. et al. The impact of pre-eclampsia definitions on the identification of adverse outcome risk in hypertensive pregnancy – analyses from the CHIPS trial (Control of Hypertension in Pregnancy Study). BJOG 128, 1373–1382 (2021).
O’Sullivan, E. P. et al. Atlantic Diabetes in Pregnancy (DIP): the prevalence and outcomes of gestational diabetes mellitus using new diagnostic criteria. Diabetologia 54, 1670–1675 (2011).
Bodmer-Roy, S., Morin, L., Cousineau, J. & Rey, E. Pregnancy outcomes in women with and without gestational diabetes mellitus according to the International Association of the Diabetes and Pregnancy Study Groups criteria. Obstet. Gynecol. 120, 746–752 (2012).
Mayo, K., Melamed, N., Vandenberghe, H. & Berger, H. The impact of adoption of the International Association of Diabetes in Pregnancy Study Group criteria or the screening and diagnosis of gestational diabetes. Am. J. Obstet. Gynecol. 212, e221–e229 (2015).
Trujillo, J. et al. Impact of the International Association of Diabetes and Pregnancy Study Groups criteria for gestational diabetes. Diabetes Res. Clin. Pract. 108, 288–295 (2015).
Ramezani Tehrani, F., Naz, M. S. G., Yarandi, R. B. & Behboudi-Gandevani, S. The impact of diagnostic criteria for gestational diabetes mellitus on adverse maternal outcomes: a systematic review and meta-analysis. J. Clin. Med. 10, 666 (2021).
Hartling, L. et al. Diagnostic thresholds for gestational diabetes and their impact on pregnancy outcomes: a systematic review. Diabet. Med. 31, 319–331 (2014).
Feldman, R. K., Tieu, R. S. & Yasumura, L. Gestational diabetes screening: the International Association of the Diabetes and Pregnancy Study Groups compared with Carpenter–Coustan screening. Obstet. Gynecol. 127, 10–17 (2016).
Saccone, G., Khalifeh, A., Al-Kouatly, H. B., Sendek, K. & Berghella, V. Screening for gestational diabetes mellitus: one step versus two step approach. A meta-analysis of randomized trials. J. Matern. Fetal Neonatal Med. 33, 1616–1624 (2020).
Brown, F. M. & Wyckoff, J. Application of one-step IADPSG versus two-step diagnostic criteria for gestational diabetes in the real world: impact on health services, clinical care, and outcomes. Curr. Diab. Rep. 17, 85 (2017).
Metzger, B. E. et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 358, 1991–2002 (2008).
Metzger, B. E. et al. Hyperglycemia and adverse pregnancy outcome study: neonatal glycemia. Pediatrics 126, e1545–e1552 (2010).
Metzger, B. E. et al. International Association of Diabetes and Pregnancy Study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33, 676–682 (2010).
O’Sullivan, J. B. & Mahan, C. M. Criteria for the oral glucose tolerance test in pregnancy. Diabetes 13, 278–285 (1964).
Carpenter, M. W. & Coustan, D. R. Criteria for screening tests for gestational diabetes. Am. J. Obstet. Gynecol. 144, 768–773 (1982).
National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 28, 1039–1057 (1979).
Behboudi-Gandevani, S., Amiri, M., Bidhendi Yarandi, R. & Ramezani Tehrani, F. The impact of diagnostic criteria for gestational diabetes on its prevalence: a systematic review and meta-analysis. Diabetol. Metab. Syndr. 11, 11 (2019).
Gillespie, P., O’Neill, C., Avalos, G. & Dunne, F. P. New estimates of the costs of universal screening for gestational diabetes mellitus in Ireland. Ir. Med. J. 105, s15–s18 (2012).
Kalra, S., Baruah, M. P., Gupta, Y. & Kalra, B. Gestational diabetes: an onomastic opportunity. Lancet Diabetes Endocrinol. 1, 91 (2013).
Moss, J. R., Crowther, C. A., Hiller, J. E., Willson, K. J. & Robinson, J. S. Costs and consequences of treatment for mild gestational diabetes mellitus–evaluation from the ACHOIS randomised trial. BMC Pregnancy Childbirth 7, 27 (2007).
Ohno, M. S., Sparks, T. N., Cheng, Y. W. & Caughey, A. B. Treating mild gestational diabetes mellitus: a cost-effectiveness analysis. Am. J. Obstet. Gynecol. 205, e281–e287 (2011).
Lowe, W. L. et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA 320, 1005–1016 (2018).
Lauring, J. R., Kunselman, A. R., Pauli, J. M., Repke, J. T. & Ural, S. H. Comparison of healthcare utilization and outcomes by gestational diabetes diagnostic criteria. J. Perinat. Med. 46, 401–409 (2018).
Knight, M. et al. Saving lives, improving mothers’ care: lessons learned to inform maternity care from the UK and Ireland Confidential Enquiries into Maternal Deaths and Morbidity 2013–15. National Perinatal Epidemiology Unit https://www.npeu.ox.ac.uk/assets/downloads/mbrrace-uk/reports/MBRRACE-UK%20Maternal%20Report%202017%20-%20Web.pdf (2017).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Institute for Health Metrics and Evaluation. The Global Burden of Disease: a critical resource for informed policymaking. IHME http://www.healthdata.org/gbd/about (2021).
Wang, W. et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population‐based study. BMC Pregnancy Childbirth 21, 364 (2021).
Alejandro, E. U. et al. Gestational diabetes mellitus: a harbinger of the vicious cycle of diabetes. Int. J. Mol. Sci. 21, 5003 (2020).
Sacks, D. A. et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Diabetes Care 35, 526–528 (2012).
Guariguata, L., Linnenkamp, U., Beagley, J., Whiting, D. R. & Cho, N. H. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res. Clin. Pract. 103, 176–185 (2014).
International Diabetes Federation. IDF Diabetes Atlas 10th edn (IDF, 2021).
McIntyre, H. D. et al. Gestational diabetes mellitus. Nat. Rev. Dis. Prim. 5, 47 (2019).
Ananth, C. V., Keyes, K. M. & Wapner, R. J. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ 347, f6564 (2013).
Ye, C. et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS ONE 9, e100180 (2014).
Ma, R. et al. Study on the descriptive epidemiology of pregnancy-induced hypertension from 1995–2000 in Jiaxing of Zhejiang province, China [Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi 26, 960–963 (2005).
Abalos, E. et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization multicountry survey on maternal and newborn health. BJOG 121, s14–s24 (2014).
de Vienne, C. M., Creveuil, C. & Dreyfus, M. Does young maternal age increase the risk of adverse obstetric, fetal and neonatal outcomes: a cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 147, 151–156 (2009).
Li, Y. et al. Maternal age and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of over 120 million participants. Diabetes Res. Clin. Pract. 162, 108044 (2020).
Laine, M. K. et al. Gestational diabetes in primiparous women–impact of age and adiposity: a register-based cohort study. Acta Obstet. Gynecol. Scand. 97, 187–194 (2018).
Schummers, L. et al. Absolute risks of obstetric outcomes risks by maternal age at first birth: a population-based cohort. Epidemiology 29, 379–387 (2018).
Lee, K. W. et al. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth 18, 494 (2018).
Eades, C. E., Cameron, D. M. & Evans, J. M. M. Prevalence of gestational diabetes mellitus in Europe: a meta-analysis. Diabetes Res. Clin. Pract. 129, 173–181 (2017).
Meazaw, M. W., Chojenta, C., Muluneh, M. D. & Loxton, D. Systematic and meta-analysis of factors associated with preeclampsia and eclampsia in sub-Saharan Africa. PLoS ONE 15, e0237600 (2020).
Bilano, V. L., Ota, E., Ganchimeg, T., Mori, R. & Souza, J. P. Risk factors of pre-eclampsia/eclampsia and its adverse outcomes in low- and middle-income countries: a WHO secondary analysis. PLoS ONE 9, e91198 (2014).
Wang, Z. et al. Maternal adiposity as an independent risk factor for pre-eclampsia: a meta-analysis of prospective cohort studies. Obes. Rev. 14, 508–521 (2013).
Shah, A., Stotland, N. E., Cheng, Y. W., Ramos, G. A. & Caughey, A. B. The association between body mass index and gestational diabetes mellitus varies by race/ethnicity. Am. J. Perinatol. 28, 515–520 (2011).
Shin, D. & Song, W. O. Prepregnancy body mass index is an independent risk factor for gestational hypertension, gestational diabetes, preterm labor, and small- and large-for-gestational-age infants. J. Matern. Fetal Neonatal Med. 28, 1679–1686 (2015).
Chu, S. Y. et al. Maternal obesity and risk of gestational diabetes mellitus. Diabetes Care 30, 2070–2076 (2007).
Hofmeyr, G. J., Lawrie, T. A., Atallah, Á. N. & Torloni, M. R. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database Syst. Rev. 10, CD001059 (2018).
Kinshella, M. W. et al. Effects of maternal nutritional supplements and dietary interventions on placental complications: an umbrella review, meta-analysis and evidence map. Nutrients 13, 472 (2021).
Hofmeyr, G. J., Manyame, S., Medley, N. & Williams, M. J. Calcium supplementation commencing before or early in pregnancy, for preventing hypertensive disorders of pregnancy. Cochrane Database Syst. Rev. 9, CD011192 (2019).
Akbari, S., Khodadadi, B., Ahmadi, S., Abbaszadeh, S. & Shahsavar, F. Association of vitamin D level and vitamin D deficiency with risk of preeclampsia: a systematic review and updated meta-analysis. Taiwan. J. Obstet. Gynecol. 57, 241–247 (2018).
Aguilar-Cordero, M. J. et al. Vitamin D, preeclampsia and prematurity: a systematic review and meta-analysis of observational and interventional studies. Midwifery 87, 102707 (2020).
Belizán, J. M., Villar, J. & Repke, J. The relationship between calcium intake and pregnancy-induced hypertension: up-to-date evidence. Am. J. Obstet. Gynecol. 158, 898–902 (1988).
Osorio-Yanez, C. et al. Risk of gestational diabetes mellitus in relation to maternal dietary calcium intake. Public Health Nutr. 20, 1082–1089 (2017).
Bao, W. et al. Prepregnancy low-carbohydrate dietary pattern and risk of gestational diabetes mellitus: a prospective cohort study. Am. J. Clin. Nutr. 99, 1378–1384 (2014).
Looman, M. et al. Pre-pregnancy dietary carbohydrate quantity and quality, and risk of developing gestational diabetes: the Australian Longitudinal Study on Women’s Health. Br. J. Nutr. 120, 435–444 (2018).
Mijatovic-Vukas, J. et al. Associations of diet and physical activity with risk for gestational diabetes mellitus: a systematic review and meta-analysis. Nutrients 10, 698 (2018).
O’Malley, E. G. et al. Maternal obesity and dyslipidemia associated with gestational diabetes mellitus (GDM). Eur. J. Obstet. Gynecol. Reprod. Biol. 246, 67–71 (2020).
Shin, D., Lee, K. W. & Song, W. O. Dietary patterns during pregnancy are associated with risk of gestational diabetes mellitus. Nutrients 7, 9369–9382 (2015).
Schoenaker, D. A., Soedamah-Muthu, S. S., Callaway, L. K. & Mishra, G. D. Prepregnancy dietary patterns and risk of developing hypertensive disorders of pregnancy: results from the Australian Longitudinal Study on Women’s Health. Am. J. Clin. Nutr. 102, 94–101 (2015).
Sahariah, S. A. et al. A daily snack containing leafy green vegetables, fruit, and milk before and during pregnancy prevents gestational diabetes in a randomized, controlled trial in Mumbai, India. J. Nutr. 146, 1453s–1460s (2016).
Schoenaker, D. A., Mishra, G. D., Callaway, L. K. & Soedamah-Muthu, S. S. The role of energy, nutrients, foods, and dietary patterns in the development of gestational diabetes mellitus: a systematic review of observational studies. Diabetes Care 39, 16–23 (2016).
Kinshella, M. W. et al. Maternal dietary patterns and pregnancy hypertension in low- and middle-income countries: a systematic review and meta-analysis. Adv. Nutr. 12, 2387–2400 (2021).
Dolatkhah, N., Hajifaraji, M. & Shakouri, S. K. Nutrition therapy in managing pregnant women with gestational diabetes mellitus: a literature review. J. Fam. Reprod. Health 12, 57–72 (2018).
Lee, C. J. et al. Risk factors for pre-eclampsia in an Asian population. Int. J. Gynaecol. Obstet. 70, 327–333 (2000).
Coonrod, D. V., Hickok, D. E., Zhu, K., Easterling, T. R. & Daling, J. R. Risk factors for preeclampsia in twin pregnancies: a population-based cohort study. Obstet. Gynecol. 85, 645–650 (1995).
Eskenazi, B., Fenster, L. & Sidney, S. A multivariate analysis of risk factors for preeclampsia. JAMA 266, 237–241 (1991).
Conde-Agudelo, A. & Belizán, J. M. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG 107, 75–83 (2000).
Ros, H. S., Cnattingius, S. & Lipworth, L. Comparison of risk factors for preeclampsia and gestational hypertension in a population-based cohort study. Am. J. Epidemiol. 147, 1062–1070 (1998).
Duckitt, K. & Harrington, D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ 330, 565 (2005).
Bartsch, E., Medcalf, K. E., Park, A. L. & Ray, J. G. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ 353, i1753 (2016).
Milne, F. et al. The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community. BMJ 330, 576–580 (2005).
Grum, T., Seifu, A., Abay, M., Angesom, T. & Tsegay, L. Determinants of pre-eclampsia/eclampsia among women attending delivery services in selected public hospitals of Addis Ababa, Ethiopia: a case control study. BMC Pregnancy Childbirth 17, 307 (2017).
Leon, L. J. et al. Preeclampsia and cardiovascular disease in a large UK pregnancy cohort of linked electronic health records. Circulation 140, 1050–1060 (2019).
Zhang, J., Troendle, J. F. & Levine, R. J. Risks of hypertensive disorders in the second pregnancy. Paediatr. Perinat. Epidemiol. 15, 226–231 (2001).
Boghossian, N. S., Albert, P. S., Mendola, P., Grantz, K. L. & Yeung, E. Delivery blood pressure and other first pregnancy risk factors in relation to hypertensive disorders in second pregnancies. Am. J. Hypertens. 28, 1172–1179 (2015).
Wang, J., Yang, W., Xiao, W. & Cao, S. The association between smoking during pregnancy and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Int. J. Gynaecol. Obstet. 157, 31–41 (2022).
Kharkova, O. A., Grjibovski, A. M., Krettek, A., Nieboer, E. & Odland, J. Ø. First-trimester smoking cessation in pregnancy did not increase the risk of preeclampsia/eclampsia: a Murmansk County Birth Registry study. PLoS ONE 12, e0179354 (2017).
Yang, Q. et al. Maternal cigarette smoking and the risk of pregnancy-induced hypertension and eclampsia. Int. J. Epidemiol. 35, 288–293 (2005).
Retnakaran, R. et al. Fetal sex and maternal risk of gestational diabetes mellitus: the impact of having a boy. Diabetes Care 38, 844–851 (2015).
Yamashita, H. et al. Fetal sex and maternal insulin resistance during mid-pregnancy: a retrospective cohort study. BMC Pregnancy Childbirth 20, 560 (2020).
Retnakaran, R. & Shah, B. R. Fetal sex and the natural history of maternal risk of diabetes during and after pregnancy. J. Clin. Endocrinol. Metab. 100, 2574–2580 (2015).
Jaskolka, D., Retnakaran, R., Zinman, B. & Kramer, C. K. Sex of the baby and risk of gestational diabetes mellitus in the mother: a systematic review and meta-analysis. Diabetologia 58, 2469–2475 (2015).
Lo, J. C. et al. Increased prevalence of gestational diabetes mellitus among women with diagnosed polycystic ovary syndrome: a population-based study. Diabetes Care 29, 1915–1917 (2006).
Seghieri, G. et al. Does parity increase insulin resistance during pregnancy? Diabet. Med. 22, 1574–1580 (2005).
Tobias, D. K., Zhang, C., van Dam, R. M., Bowers, K. & Hu, F. B. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care 34, 223–229 (2005).
Zhang, C., Solomon, C. G., Manson, J. E. & Hu, F. B. A prospective study of pregravid physical activity and sedentary behaviors in relation to the risk for gestational diabetes mellitus. Arch. Intern. Med. 166, 543–548 (2006).
Aune, D., Sen, A., Henriksen, T., Saugstad, O. D. & Tonstad, S. Physical activity and the risk of gestational diabetes mellitus: a systematic review and dose-response meta-analysis of epidemiological studies. Eur. J. Epidemiol. 31, 967–997 (2016).
Caughey, A. B., Stotland, N. E., Washington, A. E. & Escobar, G. J. Maternal ethnicity, paternal ethnicity, and parental ethnic discordance: predictors of preeclampsia. Obstet. Gynecol. 106, 156–161 (2005).
Rao, A. K., Cheng, Y. W. & Caughey, A. B. Perinatal complications among different Asian-American subgroups. Am. J. Obstet. Gynecol. 194, e39–e41 (2006).
Johnson, J. D. & Louis, J. M. Does race or ethnicity play a role in the origin, pathophysiology, and outcomes of preeclampsia? An expert review of the literature. Am. J. Obstet. Gynecol. 226, S876–S885 (2022).
Bornstein, E., Eliner, Y., Chervenak, F. A. & Grünebaum, A. Racial disparity in pregnancy risks and complications in the US: temporal changes during 2007–2018. J. Clin. Med. 9, 1414 (2020).
Anderson, N. H., Sadler, L. C., Stewart, A. W., Fyfe, E. M. & McCowan, L. M. Ethnicity, body mass index and risk of pre-eclampsia in a multiethnic New Zealand population. Aust. N. Z. J. Obstet. Gynaecol. 52, 552–558 (2012).
Campbell, S. K., Lynch, J., Esterman, A. & McDermott, R. Pre-pregnancy predictors of hypertension in pregnancy among Aboriginal and Torres Strait Islander women in north Queensland, Australia; a prospective cohort study. BMC Public. Health 13, 138 (2013).
Ghosh, G. et al. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethn. Dis. 24, 283–289 (2014).
Chang, A. L., Hurwitz, E., Miyamura, J., Kaneshiro, B. & Sentell, T. Maternal risk factors and perinatal outcomes among Pacific Islander groups in Hawaii: a retrospective cohort study using statewide hospital data. BMC Pregnancy Childbirth 15, 239 (2015).
Berkowitz, G. S., Lapinski, R. H., Wein, R. & Lee, D. Race/ethnicity and other risk factors for gestational diabetes. Am. J. Epidemiol. 135, 965–973 (1992).
Dornhorst, A. et al. High prevalence of gestational diabetes in women from ethnic minority groups. Diabet. Med. 9, 820–825 (1992).
Hedderson, M. et al. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care 35, 1492–1498 (2012).
Girgis, C. M., Gunton, J. E. & Cheung, N. The influence of ethnicity on the development of type 2 diabetes mellitus in women with gestational diabetes: a prospective study and review of the literature. ISRN Endocrinol. 2012, 341638 (2012).
Savitz, D. A., Janevic, T. M., Engel, S. M., Kaufman, J. S. & Herring, A. H. Ethnicity and gestational diabetes in New York City, 1995–2003. BJOG 115, 969–978 (2008).
Pedula, K. L. et al. Ethnic differences in gestational oral glucose screening in a large US population. Ethn. Dis. 19, 414–419 (2009).
Kragelund Nielsen, K., Andersen, G. S., Damm, P. & Andersen, A. N. Gestational diabetes risk in migrants. A nationwide, register-based study of all births in Denmark 2004 to 2015. J. Clin. Endocrinol. Metab. 105, e692–e703 (2020).
Wan, C. S. et al. Ethnic differences in prevalence, risk factors, and perinatal outcomes of gestational diabetes mellitus: a comparison between immigrant ethnic Chinese women and Australian-born Caucasian women in Australia. J. Diabetes 11, 809–817 (2019).
McDonald, R., Karahalios, A., Le, T. & Said, J. A retrospective analysis of the relationship between ethnicity, body mass index, and the diagnosis of gestational diabetes in women attending an Australian antenatal clinic. Int. J. Endocrinol. 2015, 297420 (2015).
O’Gorman, N. et al. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks gestation. Am. J. Obstet. Gynecol. 214, 103.e1–103.e12 (2016).
Yang, J. et al. Racial-ethnic differences in midtrimester maternal serum levels of angiogenic and antiangiogenic factors. Am. J. Obstet. Gynecol. 215, 359.e1–359.e9 (2016).
Abo-Elmatty, D. M. & Mehanna, E. T. MIR146A rs2910164 (G/C) polymorphism is associated with incidence of preeclampsia in gestational diabetes patients. Biochem. Genet. 57, 222–233 (2019).
Beysel, S. et al. HNF1A gene p.I27L is associated with co-existing preeclampsia in gestational diabetes mellitus. Gynecol. Endocrinol. 36, 530–534 (2020).
Dmitrenko, O. P., Karpova, N. S., Nurbekov, M. K. & Papysheva, O. V. I/D polymorphism gene ACE and risk of preeclampsia in women with gestational diabetes mellitus. Dis. Markers 2020, 8875230 (2020).
Ford, M. E. & Kelly, P. A. Conceptualizing and categorizing race and ethnicity in health services research. Health Serv. Res. 40, 1658–1675 (2005).
White, K., Lawrence, J. A., Tchangalova, N., Huang, S. J. & Cummings, J. L. Socially-assigned race and health: a scoping review with global implications for population health equity. Int. J. Equity Health 19, 25 (2020).
Mersha, T. B. & Abebe, T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum. Genomics 9, 1 (2015).
Mohsen, H. Race and genetics: somber history, troubled present. Yale J. Biol. Med. 93, 215–219 (2020).
Maglo, K. N., Mersha, T. B. & Martin, L. J. Population genomics and the statistical values of race: an interdisciplinary perspective on the biological classification of human populations and implications for clinical genetic epidemiological research. Front. Genet. 7, 22 (2016).
World Health Organization. Social determinants of health. WHO https://www.who.int/health-topics/social-determinants-of-health#tab=tab_1 (2022).
US Department of Health and Human Services. Healthy People 2030: Social Determinants of Health. US Department of Health and Human Services https://health.gov/healthypeople/objectives-and-data/browse-objectives#social-determinants-of-health (2022).
Thornburg, K. L., Boone-Heinonen, J. & Valent, A. M. Social determinants of placental health and future disease risks for babies. Obstet. Gynecol. Clin. North. Am. 47, 1–15 (2020).
Silva, L. M. et al. Low socioeconomic status is a risk factor for preeclampsia: the Generation R Study. J. Hypertens. 26, 1200–1208 (2008).
Silva, L. et al. Maternal educational level and risk of gestational hypertension: the Generation R Study. J. Hum. Hypertens. 22, 483–492 (2008).
Ross, K. M. et al. Socioeconomic status, preeclampsia risk and gestational length in black and white women. J. Racial Ethn. Health Disparities 6, 1182–1191 (2019).
Grady, S. C. & Ramírez, I. J. Mediating medical risk factors in the residential segregation and low birthweight relationship by race in New York City. Health Place. 14, 661–677 (2008).
Mayne, S. L., Yellayi, D., Pool, L. R., Grobman, W. A. & Kershaw, K. N. Racial residential segregation and hypertensive disorder of pregnancy among women in Chicago: analysis of electronic health record data. Am. J. Hypertens. 31, 1221–1227 (2018).
Boakye, E. et al. Nativity-related disparities in preeclampsia and cardiovascular disease risk among a racially diverse cohort of US women. JAMA Netw. Open 4, e2139564 (2021).
Hill-Briggs, F. et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 44, 258–279 (2020).
Haire-Joshu, D. & Hill-Briggs, F. The next generation of diabetes translation: a path to health equity. Annu. Rev. Public Health 40, 391–410 (2019).
Dixon, B., Peña, M. M. & Taveras, E. M. Lifecourse approach to racial/ethnic disparities in childhood obesity. Adv. Nutr. 3, 73–82 (2012).
Phonyiam, R. & Berry, D. C. Racial and ethnic disparities in health care and health outcomes for pregnant women with diabetes. Nurs. Women’s Health 25, 437–449 (2021).
Dode, M. A. & dos Santos, I. S. Non classical risk factors for gestational diabetes mellitus: a systematic review of the literature. Cad. Saude Publica 25, S341–S359 (2009).
Bo, S. et al. Low socioeconomic status as a risk factor for gestational diabetes. Diabetes Metab. 28, 139–140 (2002).
Bouthoorn, S. H. et al. Low-educated women have an increased risk of gestational diabetes mellitus: the Generation R Study. Acta Diabetol. 52, 445–452 (2015).
Song, L. et al. Socio-economic status and risk of gestational diabetes mellitus among Chinese women. Diabet. Med. 34, 1421–1427 (2017).
Carroll, X. et al. Socioeconomic, environmental and lifestyle factors associated with gestational diabetes mellitus: a matched case-control study in Beijing, China. Sci. Rep. 8, 8103 (2018).
Liu, J. et al. Indicators of socio-economic status and risk of gestational diabetes mellitus in pregnant women in urban Tianjin, China. Diabetes Res. Clin. Pract. 144, 192–199 (2018).
Smarr, M. M. et al. Persistent organic pollutants and pregnancy complications. Sci. Total Environ. 551–552, 285–291 (2016).
Zhang, C. et al. A prospective study of prepregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil. Steril. 103, 184–189 (2015).
Hinkle, S. N. et al. A longitudinal study of depression and gestational diabetes in pregnancy and the postpartum period. Diabetologia 59, 2594–2602 (2016).
Liu, Y., Wang, L., Wang, F. & Li, C. Effect of fine particulate matter (PM2.5) on rat placenta pathology and perinatal outcomes. Med. Sci. Monit. 22, 3274–3280 (2016).
Li, Z. et al. Impact of ambient PM(2.5) on adverse birth outcome and potential molecular mechanism. Ecotoxicol. Environ. Saf. 169, 248–254 (2019).
Saenen, N. D. et al. Lower placental leptin promoter methylation in association with fine particulate matter air pollution during pregnancy and placental nitrosative stress at birth in the ENVIRONAGE cohort. Environ. Health Perspect. 125, 262–268 (2017).
Neven, K. Y. et al. Placental promoter methylation of DNA repair genes and prenatal exposure to particulate air pollution: an ENVIRONAGE cohort study. Lancet Planet. Health 2, e174–e183 (2018).
Daniel, S. et al. Risk for preeclampsia following exposure to PM(2.5) during pregnancy. Env. Int. 156, 106636 (2021).
Yi, L., Wei, C. & Fan, W. Fine particulate matter (PM(2.5)), a risk factor of rat gestational diabetes with altered blood glucose and pancreatic GLUT2 expression. Gynecol. Endocrinol. https://doi.org/10.1080/09513590.2017.1318368 (2017).
Hu, C. Y. et al. Human epidemiological evidence about the association between air pollution exposure and gestational diabetes mellitus: systematic review and meta-analysis. Env. Res. 180, 108843 (2020).
Agrawal, S. & Yamamoto, S. Effect of indoor air pollution from biomass and solid fuel combustion on symptoms of preeclampsia/eclampsia in Indian women. Indoor Air 25, 341–352 (2015).
Rosen, E. M., Muñoz, M. I., McElrath, T., Cantonwine, D. E. & Ferguson, K. K. Environmental contaminants and preeclampsia: a systematic literature review. J. Toxicol. Env. Health B Crit. Rev. 21, 291–319 (2018).
Robledo, C. A., Romano, M. E. & Alonso-Magdalena, P. Review of current evidence on the impact of environmental chemicals on gestational diabetes mellitus. Curr. Epidemiol. Rep. 3, 51–62 (2016).
Wong, T., Ross, G. P., Jalaludin, B. B. & Flack, J. R. The clinical significance of overt diabetes in pregnancy. Diabet. Med. 30, 468–474 (2013).
Szmuilowicz, E. D., Josefson, J. L. & Metzger, B. E. Gestational diabetes mellitus. Endocrinol. Metab. Clin. North. Am. 48, 479–493 (2019).
Bellizzi, S. et al. Are hypertensive disorders in pregnancy associated with congenital malformations in offspring? Evidence from the WHO multicountry cross sectional survey on maternal and newborn health. BMC Pregnancy Childbirth 16, 198 (2016).
Wu, Y. et al. Association of maternal prepregnancy diabetes and gestational diabetes mellitus with congenital anomalies of the newborn. Diabetes Care 43, 2983–2990 (2020).
van Oostwaard, M. F. et al. Recurrence of hypertensive disorders of pregnancy: an individual patient data metaanalysis. Am. J. Obstet. Gynecol. 212, 624.e1–624.e17 (2015).
Zhao, G., Bhatia, D., Jung, F. & Lipscombe, L. Risk of type 2 diabetes mellitus in women with prior hypertensive disorders of pregnancy: a systematic review and meta-analysis. Diabetologia 64, 491–503 (2021).
Wu, R. et al. Hypertensive disorders of pregnancy and risk of cardiovascular disease-related morbidity and mortality: a systematic review and meta-analysis. Cardiology 145, 633–647 (2020).
Melchiorre, K. et al. Hypertensive disorders of pregnancy and future cardiovascular health. Front. Cardiovasc. Med. 7, 59 (2020).
Bellamy, L., Casas, J. P., Hingorani, A. D. & Williams, D. J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335, 974 (2007).
Wilson, B. J. et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ 326, 845 (2003).
Stuart, J. J. et al. Hypertensive disorders of pregnancy and 10-year cardiovascular risk prediction. J. Am. Coll. Cardiol. 72, 1252–1263 (2018).
Veerbeek, J. H. et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension 65, 600–606 (2015).
McDonald, S. D., Malinowski, A., Zhou, Q., Yusuf, S. & Devereaux, P. J. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am. Heart J. 156, 918–930 (2008).
Brown, M. C. et al. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur. J. Epidemiol. 28, 1–19 (2013).
Wu, P. et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ. Cardiovasc. Qual. Outcomes 10, e003497 (2017).
Lo, C. et al. Future cardiovascular disease risk for women with gestational hypertension: a systematic review and meta-analysis. J. Am. Heart Assoc. 9, e013991 (2020).
Giorgione, V., Ridder, A., Kalafat, E., Khalil, A. & Thilaganathan, B. Incidence of postpartum hypertension within 2 years of a pregnancy complicated by pre-eclampsia: a systematic review and meta-analysis. BJOG 128, 495–503 (2021).
Dall’Asta, A. et al. Cardiovascular events following pregnancy complicated by pre-eclampsia with emphasis on comparison between early- and late-onset forms: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 57, 698–709 (2021).
Li, J. et al. Increased risk of cardiovascular disease in women with prior gestational diabetes: a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 140, 324–338 (2018).
Kramer, C. K., Campbell, S. & Retnakaran, R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia 62, 905–914 (2019).
Carr, D. B. et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care 29, 2078–2083 (2006).
Shah, B. R., Retnakaran, R. & Booth, G. L. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 31, 1668–1669 (2008).
Gunderson, E. P. et al. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study. J. Am. Heart Assoc. 3, e000490 (2014).
Chen, L., Mayo, R., Chatry, A. & Hu, G. Gestational diabetes mellitus: its epidemiology and implication beyond pregnancy. Curr. Epidemiol. Rep. 3, 1–11 (2016).
Bellamy, L., Casas, J. P., Hingorani, A. D. & Williams, D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 373, 1773–1779 (2009).
Li, Z. et al. Incidence rate of type 2 diabetes mellitus after gestational diabetes mellitus: a systematic review and meta-analysis of 170,139 women. J. Diabetes Res. 2020, 3076463 (2020).
Rayanagoudar, G. et al. Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia 59, 1403–1411 (2016).
Benhalima, K., Lens, K., Bosteels, J. & Chantal, M. The risk for glucose intolerance after gestational diabetes mellitus since the introduction of the IADPSG criteria: a systematic review and meta-analysis. J. Clin. Med. 8, 1431 (2019).
Vounzoulaki, E. et al. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 369, m1361 (2020).
Dennison, R. A. et al. The absolute and relative risk of type 2 diabetes after gestational diabetes: a systematic review and meta-analysis of 129 studies. Diabetes Res. Clin. Pract. 171, 108625 (2021).
You, H., Hu, J., Liu, Y., Luo, B. & Lei, A. Risk of type 2 diabetes mellitus after gestational diabetes mellitus: a systematic review & meta-analysis. Indian J. Med. Res. 154, 62–77 (2021).
Bartha, J. L., Martinez-del-Fresno, P. & Comino-Delgado, R. Postpartum metabolism and autoantibody markers in women with gestational diabetes mellitus diagnosed in early pregnancy. Am. J. Obstet. Gynecol. 184, 965–970 (2001).
Sivaraman, S. C., Vinnamala, S. & Jenkins, D. Gestational diabetes and future risk of diabetes. J. Clin. Med. Res. 5, 92–96 (2013).
Gluckman, P. D., Hanson, M. A. & Buklijas, T. A conceptual framework for the developmental origins of health and disease. J. Dev. Orig. Health Dis. 1, 6–18 (2010).
Calkins, K. & Devaskar, S. U. Fetal origins of adult disease. Curr. Probl. Pediatr. Adolesc. Health Care 41, 158–176 (2011).
Vatten, L. J. et al. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet. Gynecol. 101, 529–533 (2003).
Tenhola, S., Rahiala, E., Halonen, P., Vanninen, E. & Voutilainen, R. Maternal preeclampsia predicts elevated blood pressure in 12-year-old children: evaluation by ambulatory blood pressure monitoring. Pediatr. Res. 59, 320–324 (2006).
Hoodbhoy, Z. et al. The impact of maternal preeclampsia and hyperglycemia on the cardiovascular health of the offspring: a systematic review and meta-analysis. Am. J. Perinatol. https://doi.org/10.1055/s-0041-1728823 (2021).
Pittara, T., Vyrides, A., Lamnisos, D. & Giannakou, K. Pre-eclampsia and long-term health outcomes for mother and infant: an umbrella review. BJOG 128, 1421–1430 (2021).
Pettitt, D. J., Baird, H. R., Aleck, K. A., Bennett, P. H. & Knowler, W. C. Excessive obesity in offspring of Pima Indian women with diabetes during pregnancy. N. Engl. J. Med. 308, 242–245 (1983).
Pettitt, D. J., Nelson, R. G., Saad, M. F., Bennett, P. H. & Knowler, W. C. Diabetes and obesity in the offspring of Pima Indian women with diabetes during pregnancy. Diabetes Care 16, 310–314 (1993).
Philipps, L. H. et al. The diabetic pregnancy and offspring BMI in childhood: a systematic review and meta-analysis. Diabetologia 54, 1957–1966 (2011).
Lawlor, D. A., Lichtenstein, P. & Långström, N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation 123, 258–265 (2011).
Pirkola, J. et al. Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care 33, 1115–1121 (2010).
Lawlor, D. A. et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia 53, 89–97 (2010).
Kim, S. Y., England, J. L., Sharma, J. A. & Njoroge, T. Gestational diabetes mellitus and risk of childhood overweight and obesity in offspring: a systematic review. Exp. Diabetes Res. 2011, 541308 (2011).
Maher, G. M. et al. Association of hypertensive disorders of pregnancy with risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. JAMA Psychiatry 75, 809–819 (2018).
Dachew, B. A., Mamun, A., Maravilla, J. C. & Alati, R. Pre-eclampsia and the risk of autism-spectrum disorder in offspring: meta-analysis. Br. J. Psychiatry 212, 142–147 (2018).
Nahum Sacks, K. et al. Long-term neuropsychiatric morbidity in children exposed prenatally to preeclampsia. Early Hum. Dev. 130, 96–100 (2019).
Wu, C. S. et al. Preeclampsia and risk for epilepsy in offspring. Pediatrics 122, 1072–1078 (2008).
Rowland, J. & Wilson, C. A. The association between gestational diabetes and ASD and ADHD: a systematic review and meta-analysis. Sci. Rep. 11, 5136 (2021).
van Niekerk, G., Christowitz, C. & Engelbrecht, A. M. Insulin-mediated immune dysfunction in the development of preeclampsia. J. Mol. Med. 99, 889–897 (2021).
Brownfoot, F. C. et al. Metformin as a prevention and treatment for preeclampsia: effects on soluble fms-like tyrosine kinase 1 and soluble endoglin secretion and endothelial dysfunction. Am. J. Obstet. Gynecol. 214, 356.e1–356.e15 (2016).
Dodd, J. M., Grivell, R. M., Deussen, A. R. & Hague, W. M. Metformin for women who are overweight or obese during pregnancy for improving maternal and infant outcomes. Cochrane Database Syst. Rev. 7, CD010564 (2018).
Alqudah, A. et al. Risk of pre-eclampsia in women taking metformin: a systematic review and meta-analysis. Diabet. Med. 35, 160–172 (2018).
Tarry-Adkins, J. L., Ozanne, S. E. & Aiken, C. E. Impact of metformin treatment during pregnancy on maternal outcomes: a systematic review/meta-analysis. Sci. Rep. 11, 9240 (2021).
Nascimento, I. et al. Evaluation of preeclampsia results after use of metformin in gestation: systematic review and meta-analysis. Rev. Bras. Ginecol. Obstet. 40, 713–721 (2018).
Kumasawa, K. et al. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc. Natl Acad. Sci. USA 108, 1451–1455 (2011).
Ermini, L., Post, M. & Caniggia, I. Statins, mevalonate pathway and its intermediate products in placental development and preeclampsia. Curr. Mol. Pharmacol. 10, 152–160 (2017).
Brownfoot, F. C. et al. Effects of simvastatin, rosuvastatin and pravastatin on soluble fms-like tyrosine kinase 1 (sFlt-1) and soluble endoglin (sENG) secretion from human umbilical vein endothelial cells, primary trophoblast cells and placenta. BMC Pregnancy Childbirth 16, 117 (2016).
Bauer, A. J. et al. Pravastatin attenuates hypertension, oxidative stress, and angiogenic imbalance in rat model of placental ischemia-induced hypertension. Hypertension 61, 1103–1110 (2013).
Esteve-Valverde, E. et al. Pravastatin for preventing and treating preeclampsia: a systematic review. Obstet. Gynecol. Surv. 73, 40–55 (2018).
Garrett, N. et al. Pravastatin therapy during preeclampsia prevents long-term adverse health effects in mice. JCI Insight 3, e120147 (2018).
Saleh, L. et al. Low soluble fms-like tyrosine kinase-1, endoglin, and endothelin-1 levels in women with confirmed or suspected preeclampsia using proton pump inhibitors. Hypertension 70, 594–600 (2017).
Onda, K. et al. Proton pump inhibitors decrease soluble fms-like tyrosine kinase-1 and soluble endoglin secretion, decrease hypertension, and rescue endothelial dysfunction. Hypertension 69, 457–468 (2017).
Cluver, C. A. et al. Esomeprazole to treat women with preterm preeclampsia: a randomized placebo controlled trial. Am. J. Obstet. Gynecol. 219, 388.e1–388.e17 (2018).
Tong, S. et al. Pravastatin, proton-pump inhibitors, metformin, micronutrients, and biologics: new horizons for the prevention or treatment of preeclampsia. Am. J. Obstet. Gynecol. 226, S1157–S1170 (2022).
de Alwis, N. et al. Novel approaches to combat preeclampsia: from new drugs to innovative delivery. Placenta 102, 10–16 (2020).
Rana, S., Lemoine, E., Granger, J. P. & Karumanchi, S. A. Preeclampsia: pathophysiology, challenges, and perspectives. Circ. Res. 124, 1094–1112 (2019).
Grimes, S., Bombay, K., Lanes, A., Walker, M. & Corsi, D. J. Potential biological therapies for severe preeclampsia: a systematic review and meta-analysis. BMC Pregnancy Childbirth 19, 163 (2019).
Suvakov, S. et al. Emerging therapeutic potential of mesenchymal stem/stromal cells in preeclampsia. Curr. Hypertens. Rep. 22, 37 (2020).
Yu, J., Jia, J., Guo, X., Chen, R. & Feng, L. Modulating circulating sFlt1 in an animal model of preeclampsia using PAMAM nanoparticles for siRNA delivery. Placenta 58, 1–8 (2017).
Zhang, B. et al. Placenta-specific drug delivery by trophoblast-targeted nanoparticles in mice. Theranostics 8, 2765–2781 (2018).
Ganguly, E., Hula, N., Spaans, F., Cooke, C. M. & Davidge, S. T. Placenta-targeted treatment strategies: an opportunity to impact fetal development and improve offspring health later in life. Pharmacol. Res. 157, 104836 (2020).
Farrar, D. et al. Treatments for gestational diabetes: a systematic review and meta-analysis. BMJ Open 7, e015557 (2017).
Martis, R. et al. Treatments for women with gestational diabetes mellitus: an overview of Cochrane systematic reviews. Cochrane Database Syst. Rev. 8, CD012327 (2018).
Griffith, R. J. et al. Interventions to prevent women from developing gestational diabetes mellitus: an overview of Cochrane reviews. Cochrane Database Syst. Rev. 6, CD012394 (2020).
Brown, J. et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst. Rev. 5, CD011970 (2017).
Chen, Y. et al. Effects of probiotics on blood glucose, biomarkers of inflammation and oxidative stress in pregnant women with gestational diabetes mellitus: a meta-analysis of randomized controlled trials. Med. Clin. 154, 199–206 (2020).
de Brito Alves, J. L. et al. Gut microbiota and probiotic intervention as a promising therapeutic for pregnant women with cardiometabolic disorders: present and future directions. Pharmacol. Res. 145, 104252 (2019).
Wallis, A. B., Tsigas, E. Z., Saftlas, A. F. & Sibai, B. M. Prenatal education is an opportunity for improved outcomes in hypertensive disorders of pregnancy: results from an internet-based survey. J. Matern. Fetal Neonatal Med. 26, 1565–1567 (2013).
Dijkhuis, T. E. et al. Investigating the current knowledge and needs concerning a follow-up for long-term cardiovascular risks in Dutch women with a preeclampsia history: a qualitative study. BMC Pregnancy Childbirth 20, 486 (2020).
Crowther, C. A. et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N. Engl. J. Med. 352, 2477–2486 (2005).
Klonoff, D. C. Improved outcomes from diabetes monitoring: the benefits of better adherence, therapy adjustments, patient education, and telemedicine support. J. Diabetes Sci. Technol. 6, 486–490 (2012).
de Barros, M. C., Lopes, M. A., Francisco, R. P., Sapienza, A. D. & Zugaib, M. Resistance exercise and glycemic control in women with gestational diabetes mellitus. Am. J. Obstet. Gynecol. 203, 556.e1–556.e6 (2010).
Ogu, R. N. et al. Gestational diabetes mellitus knowledge among women of reproductive age in southern Nigeria: implications for diabetes education. Int. Q. Community Health Educ. 40, 177–183 (2020).
Hussain, Z., Yusoff, Z. M. & Sulaiman, S. A. Evaluation of knowledge regarding gestational diabetes mellitus and its association with glycaemic level: a Malaysian study. Prim. Care Diabetes 9, 184–190 (2015).
Agurs-Collins, T. et al. Designing and assessing multilevel interventions to improve minority health and reduce health disparities. Am. J. Public Health 109, S86–S93 (2019).
von Dadelszen, P. et al. The Community-Level Interventions for Pre-eclampsia (CLIP) cluster randomised trials in Mozambique, Pakistan, and India: an individual participant-level meta-analysis. Lancet 396, 553–563 (2020).
Bone, J. N. et al. Economic and cost-effectiveness analysis of the Community-Level Interventions for Pre-eclampsia (CLIP) trials in India, Pakistan and Mozambique. BMJ Glob. Health 6, e004123 (2021).
Marquez, I., Calman, N. & Crump, C. Using enhanced primary care services in high-risk Latino populations to reduce disparities in glycemic control. J. Health Care Poor Underserved 29, 676–686 (2018).
Rothan, H. A. & Byrareddy, S. N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 109, 102433 (2020).
Kc, A. et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob. Health 8, e1273–e1281 (2020).
Papageorghiou, A. T. et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am. J. Obstet. Gynecol. 225, 289.e1–289.e17 (2021).
Holman, N. et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 8, 823–833 (2020).
Grasselli, G. et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 323, 1574–1581 (2020).
Goyal, P. et al. Clinical characteristics of Covid-19 in New York city. N. Engl. J. Med. 382, 2372–2374 (2020).
Myers, L. C., Parodi, S. M., Escobar, G. J. & Liu, V. X. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA 323, 2195–2198 (2020).
Murphy, H. R. Managing diabetes in pregnancy before, during, and after COVID-19. Diabetes Technol. Ther. 22, 454–461 (2020).
Author information
Authors and Affiliations
Contributions
Z.A.B. and L.J. researched data for the article. Z.A.B., L.J., K.T. and P.v.D. contributed substantially to discussion of the content. All authors wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Xilin Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, L., Tang, K., Magee, L.A. et al. A global view of hypertensive disorders and diabetes mellitus during pregnancy. Nat Rev Endocrinol 18, 760–775 (2022). https://doi.org/10.1038/s41574-022-00734-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-022-00734-y
This article is cited by
-
Effect of arsenic on the risk of gestational diabetes mellitus: a systematic review and meta-analysis
BMC Public Health (2024)
-
Atrial fibrillation considerations in the fourth trimester (postpartum period)
Journal of Interventional Cardiac Electrophysiology (2024)
-
Polygenic prediction of preeclampsia and gestational hypertension
Nature Medicine (2023)
-
Low prevalence of high blood pressure in pregnant women in Burkina Faso: a cross-sectional study
BMC Pregnancy and Childbirth (2022)