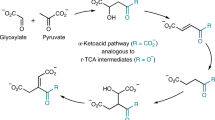
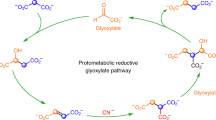
Abstract
The Strecker reaction of aldehydes is the pre-eminent pathway to explain the prebiotic origins of α-amino acids. However, biology employs transamination of α-ketoacids to synthesize amino acids which are then transformed to nucleobases, implying an evolutionary switch—abiotically or biotically—of a prebiotic pathway involving the Strecker reaction into today’s biosynthetic pathways. Here we show that α-ketoacids react with cyanide and ammonia sources to form the corresponding α-amino acids through the Bucherer–Bergs pathway. An efficient prebiotic transformation of oxaloacetate to aspartate via N-carbamoyl aspartate enables the simultaneous formation of dihydroorotate, paralleling the biochemical synthesis of orotate as the precursor to pyrimidine nucleobases. Glyoxylate forms both glycine and orotate and reacts with malonate and urea to form aspartate and dihydroorotate. These results, along with the previously demonstrated protometabolic analogues of the Krebs cycle, suggest that there can be a natural emergence of congruent forerunners of biological pathways with the potential for seamless transition from prebiotic chemistry to modern metabolism.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Burton, A. S., Stern, J. C., Elsila, J. E., Glavin, D. P. & Dworkin, J. P. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 41, 5459–5472 (2012).
Frenkel-Pinter, M., Samanta, M., Ashkenasy, G. & Leman, L. J. Prebiotic peptides: molecular hubs in the origin of life. Chem. Rev. 120, 4707–4765 (2020).
Yadav, M., Kumar, R. & Krishnamurthy, R. Chemistry of abiotic nucleotide synthesis. Chem. Rev. 120, 4766–4805 (2020).
Miller, S. L. & Van Trump, J. E. in Origin of Life: Proceedings of the Third ISSOL Meeting and the Sixth ICOL Meeting, Jerusalem, June 22-27, 1980 (ed Y. Wolman) 135–141 (D. Reidel Publishing Company, 1981).
Kitadai, N. & Maruyama, S. Origins of building blocks of life: a review. Geosci. Front. 9, 1117–1153 (2018).
Strecker, A. Ueber die künstliche Bildung der Milchsäure und einen neuen, dem Glycocoll homologen Körper. Justus Liebigs Ann. Chemie 75, 27–45 (1850). 3.
Wu, L. F. & Sutherland, J. D. Provisioning the origin and early evolution of life. Emerg. Top. Life Sci. 3, 459–468 (2019).
Harrison, S. A. & Lane, N. Life as a guide to prebiotic nucleotide synthesis. Nat. Commun. 9, 5176 (2018).
McMurry, J. & Begley, T. The Organic Chemistry of Biological Pathways (Roberts and Company, 2005).
Lazcano, A. & Miller, S. L. The origin and early evolution of life: prebiotic chemistry, the pre-RNA world, and time. Cell 85, 793–798 (1996).
Sutherland, J. D. The origin of life—out of the blue. Angew. Chem. Int. Ed. 55, 104–121 (2016).
Lazcano, A. & Miller, S. L. On the origin of metabolic pathways. J. Mol. Evol. 49, 424–431 (1999).
Hartman, H. Speculations on the origin and evolution of metabolism. J. Mol. Evol. 4, 359–370 (1975).
Forterre, P. & Gribaldo, S. The origin of modern terrestrial life. HFSP J 1, 156–168 (2007).
Orgel, L. E. The implausibility of metabolic cycles on the prebiotic Earth. PLoS Biol. 6, e18 (2008).
Krishnamurthy, R. Life’s biological chemistry: a destiny or destination starting from prebiotic chemistry? Chem. Eur. J. 24, 16708–16715 (2018).
Stubbs, R. T., Yadav, M., Krishnamurthy, R. & Springsteen, G. A plausible metal-free ancestral analogue of the Krebs cycle composed entirely of alpha-ketoacids. Nat. Chem. 12, 1016–1022 (2020).
Springsteen, G., Yerabolu, J. R., Nelson, J., Rhea, C. J. & Krishnamurthy, R. Linked cycles of oxidative decarboxylation of glyoxylate as protometabolic analogs of the citric acid cycle. Nat. Commun. 9, 91 (2018).
Yadav, M., Pulletikurti, S., Yerabolu, J. R. & Krishnamurthy, R. Cyanide as a primordial reductant enables a protometabolic reductive glyoxylate pathway. Nat. Chem. 14, 170–178 (2022).
Hafenbradl, D., Keller, M., Wächtershäuser, G. & Stetter, K. O. Primordial amino acids by reductive amination of α-oxo acids in conjunction with the oxidative formation of pyrite. Tetrahedron Lett. 36, 5179–5182 (1995).
Huber, C. & Wächtershäuser, G. Primordial reductive amination revisited. Tetrahedron Lett. 44, 1695–1697 (2003).
Barge, L. M., Flores, E., Baum, M. M., VanderVelde, D. G. & Russell, M. J. Redox and pH gradients drive amino acid synthesis in iron oxyhydroxide mineral systems. Proc. Natl Acad. Sci. 116, 4828–4833 (2019).
Wang, W. et al. Photocatalytic reversible amination of α-keto acids on a ZnS surface: implications for the prebiotic metabolism. Chem. Commun. 48, 2146–2148 (2012).
Muchowska, K. B. et al. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 1, 1716–1721 (2017).
Muchowska, K. B., Varma, S. J. & Moran, J. Synthesis and breakdown of universal metabolic precursors promoted by iron. Nature 569, 104–107 (2019).
Canavelli, P., Islam, S. & Powner, M. W. Peptide ligation by chemoselective aminonitrile coupling in water. Nature 571, 546–549 (2019).
Ashe, K. et al. Selective prebiotic synthesis of phosphoroaminonitriles and aminothioamides in neutral water. Commun. Chem. 2, 23 (2019).
Osumah, A. & Krishnamurthy, R. Diamidophosphate (DAP)—a plausible prebiotic phosphorylating reagent with a chem to biochem potential? ChemBioChem 22, 3001–3009 (2021).
Bucherer, H. T. & Fischbeck, H. Hexahydrodiphenylamine and its derivatives. J. Prakt. Chem. 140, 69–89 (1934).
Ware, E. The chemistry of the hydantoins. Chem. Rev. 46, 403–470 (1950).
Bucherer, H. T. & Lieb, V. A. Über die bildung substituierter hydantoine aus aldehyden und ketonen. Synthese von hydantoinen. J. Prakt. Chemie 141, 5–43 (1934).
Pascal R., Boiteau L. & Commeyras A. in Prebiotic Chemistry (ed Walde P.) 69–122 (Springer Berlin Heidelberg; Berlin, Heidelberg, 2005).
Zhao, H., Yu, R., Qiao, H. & Liu, C. Study on the formation of glycine by hydantoin and its kinetics. ACS Omega 5, 13463–13472 (2020).
Konnert, L., Lamaty, F., Martinez, J. & Colacino, E. Recent advances in the synthesis of hydantoins: the state of the art of a valuable scaffold. Chem. Rev. 117, 13757–13809 (2017).
Taillades, J. et al. N-Carbamoyl-α-amino acids rather than free α-amino acids formation in the primitive hydrosphere: a novel proposal for the emergence of prebiotic peptides. Orig. Life Evol. Biosph. 28, 61–77 (1998).
Rousset, A., Lasperas, M., Taillades, J. & Commeyras, A. Systemes de strecker et apparentes—XI: Formation et stabilité de l’α-carboxyaminonitrile. Intermédiaire essentiel dans la synthèse des hydantoïnes selon Bucherer–Bergs. Tetrahedron 36, 2649–2661 (1980).
Shimoyama, A. & Ogasawara, R. Dipeptides and diketopiperazines in the Yamato-791198 and Murchison carbonaceous chondrites. Orig. Life Evol. biosph. 32, 165–179 (2002).
Cooper, G. W. & Cronin, J. R. Linear and cyclic aliphatic carboxamides of the Murchison meteorite: hydrolyzable derivatives of amino acids and other carboxylic acids. Geochim. Cosmochim. Acta 59, 1003–1015 (1995).
Maltais, T. R., VanderVelde, D., LaRowe, D. E., Goldman, A. D. & Barge, L. M. Reactivity of metabolic intermediates and cofactor stability under model early Earth conditions. Orig. Life Evol. Biosph. 50, 35–55 (2020).
Yamagata, Y. et al. Prebiotic synthesis of orotic acid parallel to the biosynthetic pathway. Orig. Life Evol. Biosph. 20, 389–399 (1990).
Clay, A. P. et al. A plausible prebiotic one-pot synthesis of orotate and pyruvate suggestive of common protometabolic pathways. Angew. Chemie Int. Ed. 61, e202112572 (2022).
Basavaiah, D., Sharada, D. S. & Veerendhar, A. Organo-base mediated Cannizzaro reaction. Tetrahedron Lett. 47, 5771–5774 (2006).
Patel, B. H., Percivalle, C., Ritson, D. J., Duffy, C. D. & Sutherland, J. D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 7, 301–307 (2015).
Liu, Z. et al. Prebiotic photoredox synthesis from carbon dioxide and sulfite. Nat. Chem. 13, 1126–1132 (2021).
Ritson, D. J. A cyanosulfidic origin of the Krebs cycle. Sci. Adv. 7, eabh3981 (2021).
Cooper, G., Reed, C., Nguyen, D., Carter, M. & Wang, Y. Detection and formation scenario of citric acid, pyruvic acid, and other possible metabolism precursors in carbonaceous meteorites. Proc. Natl Acad. Sci. USA 108, 14015–14020 (2011).
Yanagawa, H., Makino, Y., Sato, K., Nishizawa, M. & Egami, F. A novel way for the formation of α-amino acids and their derivatives in an aqueous medium. Adv. Space Res. 3, 69–74 (1983).
Collins Bishop, J., Cross, S. D. & Waddell, T. G. Prebiotic transamination. Orig. Life Evol. Biosph. 27, 319–324 (1997).
Peretó, J. in Handbook of Astrobiology (ed Kolb, V.) 219–233 (CRC Press, Boca Raton, 2019).
Lauber, N., Flamm, C. & Ruiz-Mirazo, K. ‘Minimal metabolism’: a key concept to investigate the origins and nature of biological systems. BioEssays 43, 2100103 (2021).
Cooper, A. J. L., Ginos, J. Z. & Meister, A. Synthesis and properties of the alpha-keto acids. Chem. Rev. 83, 321–358 (1983).
Bachmann, S., Knudsen, K. R. & Jørgensen, K. A. Mimicking enzymatic transaminations: attempts to understand and develop a catalytic asymmetric approach to chiral α-amino acids. Org. Biomol. Chem. 2, 2044–2049 (2004).
Gibard, C., Bhowmik, S., Karki, M., Kim, E. K. & Krishnamurthy, R. Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions. Nat. Chem. 10, 212–217 (2018).
Parker, E. T., Karki, M., Glavin, D. P., Dworkin, J. P. & Krishnamurthy, R. A sensitive quantitative analysis of abiotically synthesized short homopeptides using ultraperformance liquid chromatography and time-of-flight mass spectrometry. J. Chromatogr. A 1630, 461509 (2020).
Cai, W. et al. Asymmetric biomimetic transamination of α-keto amides to peptides. Nat. Commun. 12, 5174 (2021).
Toner, J. D. & Catling, D. C. A carbonate-rich lake solution to the phosphate problem of the origin of life. Proc. Natl Acad. Sci. USA 117, 883–888 (2020).
Krissansen-Totton, J., Arney, G. N. & Catling, D. C. Constraining the climate and ocean pH of the early Earth with a geological carbon cycle model. Proc. Natl Acad. Sci. USA 115, 4105–4110 (2018).
Ferris, J. P., Joshi, P. C. & Lawless, J. G. Chemical evolution. XXIX. Pyrimidines from hydrogen cyanide. BioSystems 9, 81–86 (1977).
Miyakawa, S., Cleaves, H. J. & Miller, S. L. The cold origin of life: B. Implications based on pyrimidines and purines produced from frozen ammonium cyanide solutions. Orig. Life Evol. Biosph. 32, 209–218 (2002).
Krishnamurthy, R. Giving rise to life: transition from prebiotic chemistry to protobiology. Acc. Chem. Res. 50, 455–459 (2017).
Acknowledgements
This work was jointly supported by NSF and the NASA Astrobiology Program under the NSF Center for Chemical Evolution, CHE-1504217, NASA Exobiology grant, 80NSSC18K1300 (to R.K.) and a grant from the Simons Foundation, 327124FY19 (to R.K.). We thank L. Leman and J. Peretó for feedback on the manuscript.
Author information
Authors and Affiliations
Contributions
R.K. proposed the project. G.S. and R.K. designed and supervised research. S.P and M.Y. designed and performed the experiments and collected the data. S.P., M.Y., G.S. and R.K. analysed data. R.K. wrote the manuscript with feedback and edits from S.P., M.Y. and G.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks George Cody and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Non-interference from α-amino acids in the Bucherer–Bergs reaction.
The α-amino acids formed as products have the potential to compete and react with the starting α-ketoacids under the Bucherer-Bergs reaction conditions. Starting from pyruvate and cyanide, in the presence of glycine, only the adduct 4a formed (top pathway), and the substituted hydantoin 5f is not observed (Supplementary Figs. 56–57). When ammonia is present, the reaction is channelled towards the formation of 5-methylhydantoin 6 (bottom pathway, Supplementary Figs. 60–61). This is because while the aminonitrile adduct 4 is able to form the isocyanate intermediate 5c, the amino acid–nitrile adduct 4a cannot form the corresponding isocyanate intermediate leading to hydantoin 5f since the obligate intermediate 5e lacks the hydrogen atom necessary for the ring opening reaction (when compared to 5b)32.
Supplementary information
Supplementary Information
General experimental, experimental procedures, Supplementary Figs. 1–90, Tables 1–3, Schemes 1 and 2, references and NMR spectra.
Rights and permissions
About this article
Cite this article
Pulletikurti, S., Yadav, M., Springsteen, G. et al. Prebiotic synthesis of α-amino acids and orotate from α-ketoacids potentiates transition to extant metabolic pathways. Nat. Chem. 14, 1142–1150 (2022). https://doi.org/10.1038/s41557-022-00999-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00999-w
This article is cited by
-
C2-addition patterns emerging from acetylene and nickel sulfide in simulated prebiotic hydrothermal conditions
Communications Chemistry (2023)