Abstract
We examined whether subjectively and objectively measured sleep health composites have a relationship with heart disease. 6,820 adults (Mage = 53.4 years) from the Midlife in the United States study provided self-reported sleep characteristics and heart disease history. A smaller sample (n = 663) provided actigraphy sleep data. We tested two sleep health composites, based on self-report only and both self-report and actigraphy, across multiple sleep dimensions. We used a weighted sum approach, where higher scores indicated more sleep health problems. Modified Poisson regressions adjusted for sociodemographics and known risk factors. Having more sleep health problems was associated with a higher risk of heart disease using the self-report sleep health composite (aRR = 54%, P < .001) and the actigraphy/self-report composite (aRR = 141%, P < .001). Individual sleep dimensions of satisfaction, alertness, and efficiency (from the self-report composite) and regularity, satisfaction, and timing (from the actigraphy/self-report composite) were associated with the risk of heart disease. The effect size of each sleep health composite was larger than the individual sleep dimensions. Race moderated the association between the actigraphy/self-report sleep health composite and heart disease. There was no significant moderation by sex. Findings suggest poorer sleep health across multiple dimensions may contribute to heart disease risk among middle-aged adults.
Similar content being viewed by others
Introduction
Insufficient or poor sleep is a significant risk factor for heart disease1,2,3,4,5. Studies have mostly used single sleep measures (often focusing only on sleep duration, quality, or insomnia). However, a composite of multidimensional sleep health may be more predictive of heart disease than single sleep measures3,6,7,8. For example, an individual who has any two sleep problems simultaneously (e.g., shorter sleep duration and unsatisfactory sleep) may have a higher risk of heart disease than an individual who has only one sleep problem (e.g., shorter sleep duration alone). Examining the degree of multidimensional sleep health and its association with the risk of heart disease is important in research on middle-aged adults, as multiple sleep problems may be prevalent in this population8. Poor sleep health in middle-aged adults may lead to the loss of productivity9,10, impaired immune functioning11, increased risks of heart disease4, other illnesses in later life, and early mortality7,12,13. As sleep health is modifiable14,15, understanding multidimensional sleep health in middle adulthood may contribute to future prevention strategies aimed to mitigate the risk of heart disease, which is a leading cause of death in the United States16.
Sleep health is multifaceted and complex6,8,17. Buysse18 suggests key dimensions to define and measure multidimensional sleep health: Regularity in sleep, Satisfaction with sleep, Alertness during waking hours, Timing of sleep, Sleep Efficiency, and Sleep Duration (Ru-SATED). There is an emerging paradigm shift towards studying multidimensional sleep, instead of single indicators, and its associations with health outcomes. For example, a sleep health composite (based on self-report) is associated with multiple health outcomes in adolescents, such that poorer sleep health is related to higher depressive and anxiety symptoms, more social problems related to friends and family, and higher odds of obesity19. A composite of poorer sleep health (based on self-report only) is also associated with higher odds of depression symptoms in older women20; and poorer sleep health (based on both self-report and actigraphy) is predictive of mortality in older men21. Furthermore, a sleep health composite (based on both self-report and actigraphy) in middle adulthood has been studied in relation to cardiometabolic outcomes, such that poorer sleep health is associated with higher odds of hypertension and diabetes6.
Yet, more research during middle adulthood is needed to understand the links between multidimensional sleep health and specific pathophysiological outcomes such as heart disease. Middle adulthood spans for a longer time of life and consists of diverse (and more stressful) life experiences across work and family22. Additionally, middle adulthood is when atherosclerosis (a precursor of heart disease) develops and age-related sleep issues begin to arise23. Thus, middle adulthood may involve more and diverse sleep health issues, which can be better captured by a sleep health composite rather than by single measures of sleep8,24. Examining multidimensional sleep health in middle adulthood and its associations with the risk of heart disease may increase our understanding on the importance of maintaining good sleep health during middle adulthood for reducing the risk of a leading cause of death. Potentially diverse sleep health issues in middle adulthood also raises a question whether the predictive property of sleep health may depend on specific sleep measures used. Indeed, previous research has used different sleep measures to understand “sleep health” (e.g.25), making it difficult to provide a consistent guideline for research and practical settings. To address these gaps, we operationalize two different sleep health composites in middle-aged adults and link them to the risk of physician-diagnosed heart disease. If results are consistent between two measures, it may suggest that, rather than specific sleep measures, the combination of multiple sleep measures is more important for the risk of heart disease.
Another important thing to consider is differences by sex and race. Sex and race appear to play roles in sleep quality, sleep duration, and heart disease risk. Previous studies regarding sex differences in sleep and heart disease are inconsistent and findings differ by specific sleep dimensions. For example, men are more likely to have an earlier onset of obstructive sleep apnea, and may be at a 7–8% higher risk of heart disease compared to women17,26. However, women are more likely to report insomnia symptoms due to sex hormone differences affecting circadian rhythms27. Regarding differences by race, Black individuals are more likely to have shorter sleep duration (< 6 h per night)28,29, more sleep disturbances30, and overall worse global sleep scores compared to White counterparts31. Together, these studies suggest that it is important to consider potential differences by sex and race in the sleep health and heart disease relationship.
The current study examined the associations of multidimensional sleep health with the risk of heart disease in middle-aged adults. To assess “how many” sleep health problems co-occur within an average adult (e.g., irregular sleep timing coupled with less than 6 h of sleep), we used two independent sleep health composites–one based on self-report responses only and the other one based on both actigraphy and self-report. The two sleep health composites used different sleep variables but captured common sleep health dimensions suggested by Buysse18. We hypothesized that more sleep health problems would be associated with a higher risk of heart disease. Additionally, we tested potential differences by sex and race in sleep health, heart disease, and their interrelationships. We expected that women would have more sleep health problems (especially based on self-report) and men would have a higher risk of heart disease. We also expected that racial minorities would have more sleep health problems and a higher risk of heart disease than White individuals. In terms of differences by sex and race in the sleep health and heart disease relationship, we did not formulate specific hypotheses due to inconsistent findings in the literature. Our approach lends itself to characterizing a “sleep health” message that will be more effective in motivating the public to engage in multiple sleep health behaviors that may have synergistic effects on decreasing the risk of heart disease.
Methods
Participants and procedures
Data came from the Midlife in the United States (MIDUS) study. Comprehensive assessments of sleep measures, including self-administered questionnaires and sleep actigraphy, were captured from the second-wave sample of the study recruited between 2004–2009 (M2; N = 5,555) and from the Refresher sample recruited between 2011–2014 (MR; N = 4,593). A subset of participants from M2 and MR were invited to wear a sleep Actiwatch and complete a daily sleep diary for seven consecutive days to measure objective and subjective assessments of sleep (referred to as the Biomarker Project). Comprehensive study details can be found elsewhere32,33,34,35. Figure 1 displays the sample flowchart. Our final analytic samples were 6,820 adults who provided self-report sleep data and 663 adults who provided both self-report and actigraphy sleep data. The MIDUS study was approved by all appropriate Institutional Review Boards, and all MIDUS participants provided informed consent. The current study was exempt from the IRB review process because it used publicly available, de-identifiable data. All methods were performed in accordance with the relevant guidelines and regulations.
Measures
Sleep health composites
Guided by the Ru-SATED model, we created two sleep health composite variables to capture Regularity, Satisfaction, Alertness, Timing, Efficiency, and Duration18–one composite was captured using self-reported sleep variables and the other using both actigraphy and self-report sleep variables. The self-report sleep health composite captured five of the six Ru-SATED dimensions (not sleep timing), and the actigraphy/self-report sleep health composite captured all six dimensions in the Ru-SATED model (see Table 1).
The use of these two different composites was to see whether the proposed relationship between multidimensional sleep health and heart disease would be replicated across different measures assessing sleep health. In the two sleep health composites, each dimension was measured using different sleep measures, depending on the relevance and data availability. For example, the self-report regularity dimension was captured by sleep debt, which refers to a significant gap between weekday sleep and weekend sleep36. Sleep debt is closely related to jet lag or circadian rhythm misalignment37, which refers to irregularity in sleep behavior. For the self-report alertness dimension, we assessed nap frequency as it was the only available survey measure related to lack of alertness. Frequent naps (> 2 naps per week) have been found to be associated with incident cardiovascular events38.
Each of the composites was created using a weighted sum. This approach is optimized for accurate prediction because individual sleep dimensions may be differentially associated with the risk of heart disease. We first ran unadjusted models regressing the risk of heart disease on all the sleep health dimensions simultaneously. We used z-scores of the sleep dimensions in order to allow for comparability across sleep dimensions measured on different scales. Next, we extracted the beta coefficients from this model and inputted them into an equation where all indicators were summed based on their weights (see Table 2). Higher weighted values indicated that the sleep dimension was more important for determining the risk of heart disease. We also conducted a sensitivity check with unweighted composites which assume all sleep health features contribute equally based on pre-established cut points (see Table 3 for how we created these measures).
Diagnoses of heart disease
Participants were asked, “Have you ever had heart trouble suspected or confirmed by a doctor?” and “Have you ever had a severe pain across the front of your chest lasting half an hour or more?” If participants responded “yes” to the first question, then a follow-up question asked, “What was the diagnosis?” If participants responded “yes” to the second question then a follow-up question asked, “Which did the doctor say it was?” For both follow-up questions, the lists of possible diagnoses were consistent and included: (1) heart attack; (2) angina; (3) high blood pressure; (4) valve disease, mitrovalve prolapse, aortic insufficiency, bicuspid aortic valve; (5) hole in heart, atrial septal defect, ventricular septal defect; (6) blocked/closed artery, coronary artery disease, coronary heart disease, ischemia; (7) irregular/fast heartbeat, arrhythmia; heart murmur; (8) heart failure, congestive heart failure, enlarged heart; and/or (9) other. We excluded high blood pressure as it is a risk factor of heart disease rather than a heart disease condition39. “Yes” responses to any of the heart disease diagnoses were coded as having a diagnosis of heart disease (= 1 vs. 0 = no heart disease).
Covariates
We controlled for sociodemographics and known heart disease risk factors including age, sex, race/ethnicity, education, work status, BMI, diabetes, hypertension, smoking status, depression, anxiety, and the month data collection occurred since a previous study found that sleep complaints varied across seasons40. Depression and anxiety were measured using the World Mental Health Organization’s Composite International Diagnostic Interview Short Form41. In the depression and anxiety questionnaires, sleep-related items were removed. Additional analyses were conducted controlling for family history of heart disease and physical activity; both of which were only available in the Biomarker Project. Family history of heart disease was assessed by asking participants, “Has anyone in your immediate family (blood relative only) had heart disease?” Physical activity was assessed by asking participants, “Do you engage in regular exercise, or activity, of any type for 20 min or more at least 3 times/week?” Response options for both questions were coded, 0 = No and 1 = Yes.
Statistical analyses
We used modified Poisson regression models with robust variance estimation in SAS v9.4 procedure GENMOD to test the associations of each sleep health composite with the risk of heart disease. This method allows for estimation of relative risks (RR; adjusted relative risks: aRR) and is recommended when the prevalence of outcome is > 10%42,43. The self-report only and actigraphy/self-report sleep health composites were included in separate models. To test differences by sex (men vs. women) and race (non-Hispanic Black, all other races, vs. non-Hispanic White), we used chi-squared tests, t-tests, and interaction terms between sleep health composites and each of sex and race in separate models. Continuous covariates were z-scored to improve interpretability in relation to each sleep health composite (i.e., weighted sum of z-scores). Significance was determined by using two-tailed test with a p-value of <.05.
Results
Sample characteristics and descriptive statistics are shown in Table 4. The correlations among all individual sleep variables ranged from ± 0.01 to 0.62, meaning that they were related but had unique variances. The correlation between the two sleep health composites indicated that they were moderately related (r = 0.35, P <.001).
Results from a fully adjusted modified Poisson regression models showed a significant association of the self-report sleep health composite with the risk of heart disease. Each unit increase in poor sleep health was associated with 54% higher risk of heart disease (B = 0.43, SE = 0.09, 95% CI [0.26, 0.60], aRR = 1.54, P < .001) (Fig. 2, Panel 1). For the actigraphy/self-report sleep health composite, each unit increase in poor sleep health was associated with 141% higher risk of heart disease (B = 0.88, SE = 0.22, 95% CI [0.44, 1.32], aRR = 2.41, P < .001), after adjusting for all covariates (Fig. 2, Panel 2).
As a sensitivity check, we repeated the analyses using unweighted sleep health composites. Results were generally consistent but weaker. For the self-report unweighted composite, each additional dimension of poor sleep health was associated with 14% higher risk of heart disease (B = 0.13, SE = 0.03, 95% CI [0.08, 0.18], aRR = 1.14, P < .001) (Fig. 3, Panel 1). For the actigraphy/self-report unweighted composite, the association between poor sleep health and the risk of heart disease was in the expected direction but did not reach statistical significance (B = 0.20, SE = 0.11, 95% CI [− 0.02, 0.42], aRR = 1.22, P = .073) (Fig. 3, Panel 2).
Differences by sex and race
There were significant differences in sleep health composites and heart disease by sex and race (Supplementary Fig. S1). Compared to men, women had slightly more sleep health problems based on the self-report (but no sex difference in the actigraphy/self-report sleep health composite). Men were more likely to have heart disease compared to women. However, sex did not moderate the association between sleep health composites and the risk of heart disease.
Turning to racial differences, non-Hispanic Black individuals had the highest number of sleep health problems, followed by all other races, and then non-Hispanic White individuals. This was observed in both the self-report and actigraphy/self-report sleep health composites. Race was significantly associated with heart disease, only in the actigraphy/self-report sample. Non-Hispanic Black individuals had the highest prevalence, followed by non-Hispanic White individuals, and other races. There was one significant moderation by race in the association between the actigraphy/self-report sleep health composite and heart disease. Compared to non-Hispanic White individuals, those with all other races exhibited a weaker association between the actigraphy-self-report sleep health composite and the risk of heart disease (B = − 5.83, SE = 1.77, 95% CI [− 9.30, − 2.37], P = .001). See Fig. 4 for the nature of this interaction and slope estimates. For non-Hispanic Whites, more sleep health problems were associated with significantly higher risk of heart disease. This was similar for non-Hispanic Blacks with no difference to non-Hispanic Whites (B = − 0.60, SE = 0.42, 95% CI [− 1.42, 0.22], P = .151). However, for those with other races, the slope was not significant. Race did not moderate the association between the self-report sleep health composite and the risk of heart disease. See Supplementary Table S1 for results from models stratified by sex or by race. Effect sizes of sleep health composites seemed to vary by groups.
Moderation by race in the association between actigraphy/self-report sleep health composite and the risk of heart disease. Note. Compared to non-Hispanic Whites, those with all other races exhibited a weaker association between the actigraphy-self-report sleep health composite and the risk of heart disease (B = − 5.83, SE = 1.77, 95% CI [− 9.30, − 2.37], P = .001). The slope for Non-Hispanic Whites was: B = 3.51, SE = 0.34, 95% CI [1.81, 6.81], P < .001. The slope for Non-Hispanic Blacks was: B = 1.93, SE = 0.27, 95% CI [1.93, 1.13], P = .016. The slope for all other races was: B = 1.06, SE = 0.62, 95% CI [0.31, 3.56], P = .930. There was no significant difference between non-Hispanic Blacks and non-Hispanic Whites (B = − 0.60, SE = 0.42, 95% CI [− 1.42, 0.22], P = .151). The model adjusted for all covariates.
Supplemental analyses
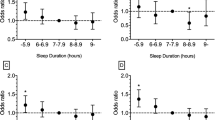
All results were consistent with or without history of stroke in the heart disease outcome (results not shown, but available upon request). We also compared the effect sizes of individual sleep health dimensions in fully adjusted models (Supplementary Fig. S2) vs. the found effect of a sleep health composite. Among the self-report sleep health dimensions, satisfaction (aRR = 0.14, 95% CI [0.03, 0.09], P < .001), alertness (aRR = 0.05, 95% CI [0.004, 0.10], P = .033), and efficiency (aRR = 0.08, 95% CI [0.04, 0.13], P < .001) were independently associated with heart disease. Regularity and duration dimensions were not significantly associated with heart disease. For the actigraphy/self-report sleep health dimensions, regularity (aRR = 0.17, 95% CI [0.04, 0.30], P = .009), satisfaction (aRR = 0.20, 95% CI [0.03, 0.37], P = .024) and timing (aRR = 0.24, 95% CI [0.11, 0.36], P < .001) dimensions were independently associated with heart disease. Overall, the effect sizes of the significant individual dimensions were smaller than that of the sleep health composite.
Next, we repeated our analysis with the self-report sleep health composite in the smaller sample that provided both actigraphy and self-report sleep data. The previously significant effect of self-report sleep health composite became non-significant (B = 0.14, SE = 0.09, 95% CI [− 0.04, 0.32], aRR = 1.15, P = .137). Lastly, we compared results before and after controlling for family history of heart disease and physical activity. Results were unchanged with the self-report composite (B = 0.40, SE = 0.18, 95% CI [0.06, 0.75], aRR = 1.49, P = .022, n = 1850) and with the actigraphy/self-report composite (B = 0.91, SE = 0.23, 95% CI [0.46, 1.36], aRR = 2.48, P < .001, n = 645).
Discussion
The current study reveals that having more sleep health problems may increase the risk of heart disease in middle adulthood. Results were consistent across our two models that used different sleep measures (i.e., self-report only and both actigraphy and self-report) and different samples. There were sex and race differences in each of sleep health and heart disease, however, the relationship between sleep health and heart disease did not differ by sex or race. Strengths of this study include the holistic assessment of sleep health using two different composites and replication of findings across different measures and samples. Findings show the utility of assessing multidimensional sleep health in predicting the risk of heart disease.
Our findings were similar to Brindle and colleagues6, who created a sleep health composite including self-reported measures of daytime alertness, quality, and timing, as well as actigraphy-based measures of regularity, efficiency, and duration. The current study extended this previous effort using a different approach. Our weighted composite approach is optimized for accurate prediction of heart disease and could be used in future studies as the regression weights were derived from a relatively large and representative sample of U.S. middle-aged adults. In our sensitivity analysis, we compared results with an unweighted sum composite, which assumes all sleep health dimensions contribute equally to the risk of heart disease. Results were generally consistent between these two approaches, with smaller effect sizes in the unweighted approach. The unweighted approach was similarly used in Brindle and colleagues6. yet our method is further distinguished by the use of a priori cutoffs established by the sleep expert panels6,8,18,38,44 that has high potential for replication across studies. For example, in our method using a priori cutoff for the sleep satisfaction/quality dimension, responses of “fairly bad” or “very bad” (vs. fairly good or very good) were identified as poor sleep quality, whereas Brindle and colleagues6 used ≥ 2.8 as an empirical cutoff that indicates poor sleep quality in their study sample. In other sleep health dimensions such as timing, efficiency, and duration, we used cutoffs that were used and validated by many studies to indicate poor sleep (e.g., < 85% for low sleep efficiency; see Table 3). Using their unweighted approach, Brindle and colleagues6 found that those with poorer sleep health were at 10% higher odds of cardiometabolic morbidity (P = .04). In our study, the risk of heart disease was 14% higher as a function of the unweighted self-report sleep health composite (P < .001). When we used our weighted approach, the detection of heart disease risk was much higher, such as 54% higher risk as a function of the self-report composite and 141% higher risk as a function of the actigraphy/self-report composite. Differences in the estimated risks may be attributed to differences in the samples as well as in the measures of sleep health and cardiovascular outcomes. Our supplemental results also showed that the significant effect of the self-report sleep health composite became non-significant when tested in the smaller actigraphy/self-report sample and the effect size was also much smaller than that of the actigraphy/self-report composite. Thus, when possible, using the actigraphy/self-report composite may increase the prediction of heart disease.
There were differences in the prevalence of heart disease and sleep health problems by sex and race, which was consistent with previous studies28,30,31. The link between sleep health and heart disease was not moderated by sex. The lack of moderation by sex may suggest that the relationship between multidimensional sleep health and heart disease is universal across men and women. There was a significant moderation by race in the association between actigraphy/self-report sleep health composite and heart disease, showing that the association was weaker for other racial minorities (including Native American, Asian, Native Hawaiian or Pacific Islander, and Hispanic) compared to non-Hispanic Whites. This may relate to a higher estimated risk of heart disease in other races regardless of their sleep health problems. We did not find a difference between non-Hispanic Blacks and Whites in the link between sleep health and heart disease; for both groups, having more sleep health problems significantly increased the risk of heart disease. However, note that effect sizes of sleep health composites varied by demographic sub-groups (Supplementary Table S1), suggesting a need to test potential moderation by these characteristics in a more diverse sample of adults. In particular, in this study, we lumped all other races together due to small % of the sub-groups (e.g., < 1% were Asian; see Table 4). Future studies could sample more individuals with other races to examine potential differences within this group. Examining potential differences in sleep health and heart disease by background characteristics is important because this helps identify at-risk groups.
Similar to previous research, we found that single sleep dimensions were associated with heart disease risk. However, the effect sizes of the individual sleep dimensions were weaker than those of sleep health composites that considered multiple sleep dimensions. Across the models, satisfaction dimension was consistently associated with the risk of heart disease, adjusting for other sleep dimensions. Previous studies found that insomnia1,3,45 and overall global sleep scores31 were related to increased risk of heart disease. Our findings further showed that the associations of these satisfaction measures with heart disease were independent of other sleep dimensions. Further, the associations were better understood when considering other sleep dimensions that may interact with sleep satisfaction. Note also that sleep duration (either based on self-report or actigraphy/self-report) was not significantly associated with heart disease. This is surprising given the reported associations of short or long sleep duration with multiple health outcomes4,13,46. This may be related to the characteristics of our sample, as most participants (78% in self-report sample; 56% in actigraphy/self-report sample) reported optimal sleep duration (≥ 6 & ≤ 8 h per night). It may also be that we examined independent association of sleep duration with heart disease (after controlling for other sleep dimensions), whereas other studies examined sleep duration as an isolated characteristic and did not adjust for potential shared variance with other sleep dimensions. Overall, this study clearly shows the importance of considering “co-exiting sleep health problems” in capturing the risk of heart disease. Our approach examining “how many” sleep health problems offers a comprehensive assessment of sleep health on one hand and the opportunity to better predict health risks on the other hand.
There are some limitations to consider. First, the current study is cross-sectional, which limits our ability to examine the effects of sleep health on heart disease risk over time. A future direction of this work is to analyze these associations longitudinally. Another limitation is the unbalanced sample sizes between our two models (6820 vs. 663), although it seems challenging to collect actigraphy sleep data in a larger sample. Further, the self-report sleep health composite could not capture sleep timing, as the larger survey did not include questions on bedtime and wake time. For other sleep dimensions, although we used the common sleep health framework18 to operationalize the two sleep health composites, specific sleep variables to capture each dimension may not be comparable between the composites and this is what we intended. Also, in the self-report sleep health composite, insomnia symptoms used to capture the satisfaction dimension may not coincide with the original conceptualization. More research is needed to examine where subjective insomnia symptoms fit into the sleep health dimensions theoretically and empirically. Another limitation is the lack of data available on sleep apnea, snoring, and bedtime use of electronic devices, all of which may influence one’s sleep health. Lastly, the majority of the MIDUS sample were White individuals and had higher education. Future studies could use more socioeconomically diverse samples, as socioeconomic status is closely linked to race47 and those with lower socioeconomic status are known to have more sleep complaints.
The current study shows the importance of considering “co-existing sleep health problems” within an individual to assess the risk of heart disease. Findings revealed having more sleep health problems may increase the risk of heart disease in middle adulthood. Results were consistent between two independent samples using different sleep health composites (using self-report only and both actigraphy and self-report). Despite known differences in the prevalence of sleep and heart disease by sex and race, the association between sleep health and the risk of heart disease did not generally differ by sex and race in our study. The findings highlight the importance and utility of assessing multidimensional sleep health in predicting the risk of heart disease and potentially other health outcomes.
Data availability
Data and documentation for all MIDUS projects are available to other researchers at the Inter-university Consortium for Political and Social Research (ICPSR). In addition to the publicly-available data at ICPSR, a MIDUS-Colectica Portal (midus.colectica.org) contains rich searchable metadata, links to helpful documentation, and the ability to download customized datasets.
References
Khan, M. S. & Aouad, R. The effects of insomnia and sleep loss on cardiovascular disease. Sleep Med. Clin. 12, 167–177 (2017).
Huang, T., Mariani, S. & Redline, S. Sleep irregularity and risk of cardiovascular events: The multi-ethnic study of atherosclerosis. J. Am. Coll. Cardiol. 75, 991–999 (2020).
Bertisch, S. M. et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep heart health study. Sleep 41, 1–9 (2018).
Lao, X. Q. et al. Sleep quality, sleep duration, and the risk of coronary heart disease: A prospective cohort study with 60,586 adults. J. Clin. Sleep Med. 14, 109–117 (2018).
Cappuccio, F. P., Cooper, D., Delia, L., Strazzullo, P. & Miller, M. A. Sleep duration predicts cardiovascular outcomes: A systematic review and meta-analysis of prospective studies. Eur. Heart J. 32, 1484–1492 (2011).
Brindle, R. C., Yu, L., Buysse, D. J. & Hall, M. H. Empirical derivation of cutoff values for the sleep health metric and its relationship to cardiometabolic morbidity: Results from the Midlife in the United States (MIDUS) study. Sleep 42, 1–9 (2019).
Wallace, M. L. et al. Multidimensional sleep and mortality in older adults: A machine-learning comparison with other risk factors. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 74, 1903–1909 (2019).
Lee, S. & Lawson, K. M. Beyond single sleep measures: A composite measure of sleep health and its associations with psychological and physical well-being in adulthood. Soc. Sci. Med. 274, 113800 (2021).
Stimpfel, A. W. et al. Nurses’ sleep, work hours, and patient care quality, and safety. Sleep Health 6, 314–320 (2020).
Lian, Y. et al. Associations between insomnia, sleep duration and poor work ability. J. Psychosom. Res. 78, 45–51 (2015).
Irwin, M. R. & Opp, M. R. Sleep health: Reciprocal regulation of sleep and innate immunity. Neuropsychopharmacology 42, 129–155 (2017).
Kronholm, E., Laatikainen, T., Peltonen, M., Sippola, R. & Partonen, T. Self-reported sleep duration, all-cause mortality, cardiovascular mortality and morbidity in Finland. Sleep Med. 12, 215–221 (2011).
Kwok, C. S. et al. Self-reported sleep duration and quality and cardiovascular disease and mortality: A dose-response meta-analysis. J. Am. Heart Assoc. 7, 1–26 (2018).
Ohayon, M. et al. National Sleep Foundation’s sleep quality recommendations: first report. Sleep Health 3, 6–19 (2017).
Mead, M. P. & Irish, L. A. Application of health behaviour theory to sleep health improvement. J. Sleep Res. 29, 1–13 (2020).
World Health Organization Global Health Estimates. The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (2020).
Buxton, O. M. et al. Sleep health and predicted cardiometabolic risk scores in employed adults from two industries. J. Clin. Sleep Med. 14, 371–383 (2018).
Buysse, D. J. Sleep health: Can we define It? does it matter?. Sleep 37, 9–17 (2014).
Dong, L., Martinez, A. J., Buysse, D. J. & Harvey, A. G. A composite measure of sleep health predicts concurrent mental and physical health outcomes in adolescents prone to eveningness. Sleep Health 5, 166–174 (2019).
Furihata, R. et al. An aggregate measure of sleep health is associated with prevalent and incident clinically significant depression symptoms among community-dwelling older women. Sleep 40, 2 (2017).
Wallace, M. L. et al. Which sleep health characteristics predict all-Cause mortality in older men? An application of flexible multivariable approaches. Sleep 41, 2 (2018).
Lachman, M. E. Development in midlife. Annu. Rev. Psychol. 55, 305–331 (2004).
Ohayon, M. M., Carskadon, M. A., Guilleminault, C. & Vitiello, M. V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27, 1255–1273 (2004).
Xiao, Q., Blot, W. J. & Matthews, C. E. Weekday and weekend sleep duration and mortality among middle-to-older aged White and Black adults in a low-income southern US cohort. Sleep Health 5, 521–527 (2019).
Knutson, K. L. et al. The national sleep foundation’s sleep health index. Sleep Health 3, 234–240 (2017).
Fietze, I. et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences–Results of SHIP-Trend. J. Sleep Res. 28, e12770 (2019).
Merz, A. A. & Cheng, S. Sex differences in cardiovascular ageing. Heart 102, 825–831 (2016).
Whinnery, J., Jackson, N., Rattanaumpawan, P. & Grandner, M. A. Short and long sleep duration associated with race/ethnicity, sociodemographics, and Socioeconomic position. Sleep 37, 601–611 (2014).
Hall, M. H., Brindle, R. C. & Buysse, D. J. Sleep and cardiovascular disease: Emerging opportunities for psychology. Am. Psychol. 73, 994–1006 (2018).
Egan, K. J., Knutson, K. L., Pereira, A. C. & von Schantz, M. The role of race and ethnicity in sleep, circadian rhythms and cardiovascular health. Sleep Med. Rev. 33, 70–78 (2017).
Chung, J. et al. Racial-ethnic differences in actigraphy, questionnaire, and polysomnography indicators of healthy sleep: The Multi-Ethnic Study of Atherosclerosis. Am. J. Epidemiol. 2, 232 (2021).
Brim, O. G., Ryff, C. D. & Kessler, R. C. The MIDUS National Survery: An Overview. In How Healthy Are We? A National Study of Well-Being at Midlife (ed. John, D.) 1–36 (The University of Chicago Press, 2004).
Weinstein, M., Ryff, C. D. & Seeman, T. E. Midlife in the United States (MIDUS Refresher): Biomarker Project, 2012–2016. https://www.icpsr.umich.edu/web/ICPSR/studies/36901 (2019).
Love, G. D., Seeman, T. E., Weinstein, M. & Ryff, C. D. Bioindicators in the MIDUS national study: Protocol, measures, sample, and comparative context. J. Aging Health 22, 1059–1080 (2010).
Ryff, C. D., Carr, D. S. & Coe, C. Midlife in the United States Midlife in the United States ( MIDUS 2 ), 2004–2006. 2004–2006 (2006).
Van Dongen, H. P. A., Rogers, N. L. & Dinges, D. F. Sleep debt: Theoretical and empirical issues. Sleep Biol. Rhythms 1, 5–13 (2003).
Fischer, D., Vetter, C., Oberlinner, C., Wegener, S. & Roenneberg, T. A unique, fast-forwards rotating schedule with 12-h long shifts prevents chronic sleep debt. Chronobiol. Int. 33, 98–107 (2016).
Häusler, N., Haba-Rubio, J., Heinzer, R. & Marques-Vidal, P. Association of napping with incident cardiovascular events in a prospective cohort study. Heart 105, 1793–1798 (2019).
Kannel, W. B. Blood pressure as a cardiovascular risk factor. JAMA 275, 1571 (1996).
Titova, O. E., Lindberg, E., Elmståhl, S., Lind, L. & Benedict, C. Seasonal variations in sleep duration and sleep complaints: A Swedish cohort study in middle-aged and older individuals. J. Sleep Res. https://doi.org/10.1111/jsr.13453 (2021).
Kessler, R. C., Andrews, G., Mroczek, D., Ustun, B. & Wittchen, H.-U. The world health organization composite international diagnostic interview short-form (CIDI-SF). Int. J. Methods Psychiatric Res. 7, 171–185 (1998).
Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 159, 702–706 (2004).
Zhang, J. & Yu, K. F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. J. Am. Med. Assoc. 280, 1690–1691 (1998).
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989).
Canivet, C., Nilsson, P. M., Lindeberg, S. I., Karasek, R. & Östergren, P. O. Insomnia increases risk for cardiovascular events in women and in men with low socioeconomic status: A longitudinal, register-based study. J. Psychosom. Res. 76, 292–299 (2014).
Grandner, M. A., Chakravorty, S., Perlis, M. L., Oliver, L. & Gurubhagavatula, I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med. 15, 42–50 (2015).
Grandner, M. A. Social-ecological model of sleep health. In Sleep and Health 45–53 (Elsevier, 2019). https://doi.org/10.1016/B978-0-12-815373-4.00005-8.
Acknowledgements
Since 1995 the Midlife in the United States Study has been funded by the following: John D. and Catherine T. MacArthur Foundation Research Network, National Institute on Aging (P01-AG020166), and National institute on Aging (U19-AG051426). The larger MIDUS study protocol was approved by the University of Wisconsin-Madison Institutional Review Board (IRB); the current study was exempt from an IRB review because we used publicly available, de-identifiable data. Written informed consent was received for all MIDUS participants.
Author information
Authors and Affiliations
Contributions
S.L. conceived the idea. S.L. and C.X.M. conduct the analyses and drafted the manuscript. M.L.W. advised the analyses. All authors (R.A., D.M.A., O.M.B., and S.R.P.) substantially contributed to the review and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
All authors have no conflicts of interests related to the material presented. Soomi Lee discloses that she received a grant from NIH/National Institute on Aging (R56AG065251). Outside of the current work, O.M.B. received subcontract grants to Pennsylvania State University from Proactive Life (formerly Mobile Sleep Technologies) doing business as SleepSpace (National Science Foundation grant #1,622,766 and NIH/National Institute on Aging Small Business Innovation Research Program R43AG056250, R44 AG056250), honoraria/travel support for lectures from Boston University, Boston College, Tufts School of Dental Medicine, Harvard Chan School of Public Health, New York University, and Allstate®, consulting fees from Sleep Number, and an honorarium for his role as the Editor-in-Chief of Sleep Health (sleephealthjournal.org). Also outside the current work, M.L.W. provides statistical consulting for Noctem, Health Rhythms, and Sleep Number.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, S., Mu, C.X., Wallace, M.L. et al. Sleep health composites are associated with the risk of heart disease across sex and race. Sci Rep 12, 2023 (2022). https://doi.org/10.1038/s41598-022-05203-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-05203-0
This article is cited by
-
Hard Work Makes It Hard to Sleep: Work Characteristics Link to Multidimensional Sleep Health Phenotypes
Journal of Business and Psychology (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.