Abstract
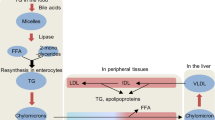
Lipids entering the gastrointestinal tract include dietary lipids (triacylglycerols, cholesteryl esters and phospholipids) and endogenous lipids from bile (phospholipids and cholesterol) and from shed intestinal epithelial cells (enterocytes). Here, we comprehensively review the digestion, uptake and intracellular re-synthesis of intestinal lipids as well as their packaging into pre-chylomicrons in the endoplasmic reticulum, their modification in the Golgi apparatus and the exocytosis of the chylomicrons into the lamina propria and subsequently to lymph. We also discuss other fates of intestinal lipids, including intestinal HDL and VLDL secretion, cytosolic lipid droplets and fatty acid oxidation. In addition, we highlight the applicability of these findings to human disease and the development of therapeutics targeting lipid metabolism. Finally, we explore the emerging role of the gut microbiota in modulating intestinal lipid metabolism and outline key questions for future research.
Key points
Dietary lipids are digested and are taken up by enterocytes for re-esterification and packaging into pre-chylomicrons in the endoplasmic reticulum, trafficked to the Golgi and then secreted for transport in the lymphatic system.
Specific proteins and enzymes are involved in this complex process; when deficient, human diseases characterized by defective lipid and fat-soluble vitamin absorption, such as abetalipoproteinaemia and chylomicron storage disease, occur.
Cytoplasmic lipid droplets are multiprotein-coated structures that serve as dynamic triacylglycerol storage pools in the enterocyte and are involved in several aspects of enterocyte lipid metabolism.
Pharmacotherapy targeted to specific proteins and/or molecules involved in the absorptive process, such as luminal lipases, bile acids, NPC1L1, MGAT2, DGAT1 and MTP, could be used to treat diet-induced obesity and its associated complications.
Studies have suggested that the gut microbiome in the small intestine has a role in regulating host metabolism and the response to dietary lipids.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kohan, A. B., Yoder, S. M. & Tso, P. Using the lymphatics to study nutrient absorption and the secretion of gastrointestinal hormones. Physiol. Behav. 105, 82–88 (2011).
Tso, P. & Balint, J. A. Formation and transport of chylomicrons by enterocytes to the lymphatics. Am. J. Physiol. 250, G715–G726 (1986).
D’Aquila, T., Hung, Y. H., Carreiro, A. & Buhman, K. K. Recent discoveries on absorption of dietary fat: presence, synthesis, and metabolism of cytoplasmic lipid droplets within enterocytes. Biochim. Biophys. Acta 1861, 730–747 (2016).
Xiao, C., Stahel, P., Carreiro, A. L., Buhman, K. K. & Lewis, G. F. Recent advances in triacylglycerol mobilization by the gut. Trends Endocrinol. Metab. 29, 151–163 (2018).
Hussain, M. M. Intestinal lipid absorption and lipoprotein formation. Curr. Opin. Lipidol. 25, 200–206 (2014).
Welty, F. K. Hypobetalipoproteinemia and abetalipoproteinemia. Curr. Opin. Lipidol. 25, 161–168 (2014).
Ferreira, H. et al. Chylomicron retention disease: a description of a new mutation in a very rare disease. Pediatr. Gastroenterol. Hepatol. Nutr. 21, 134–140 (2018).
Shiau, Y. F. et al. Intestinal triglycerides are derived from both endogenous and exogenous sources. Am. J. Physiol. 248, G164–G169 (1985).
Bernback, S., Blackberg, L. & Hernell, O. Fatty acids generated by gastric lipase promote human milk triacylglycerol digestion by pancreatic colipase-dependent lipase. Biochim. Biophys. Acta 1001, 286–293 (1989).
Hamosh, M. et al. Fat digestion in the newborn. Characterization of lipase in gastric aspirates of premature and term infants. J. Clin. Invest. 67, 838–846 (1981).
Tso, P. Gastrointestinal digestion and absorption of lipid. Adv. Lipid Res. 21, 143–186 (1985).
Watkins, J. B. Lipid digestion and absorption. Pediatrics 75, 151–156 (1985).
Phan, C. T. & Tso, P. Intestinal lipid absorption and transport. Front. Biosci. 6, D299–D319 (2001).
Einarsson, K. et al. Bile acid sequestrants: mechanisms of action on bile acid and cholesterol metabolism. Eur. J. Clin. Pharmacol. 40, S53–S58 (1991).
Chow, S. L. & Hollander, D. A dual, concentration-dependent absorption mechanism of linoleic acid by rat jejunum in vitro. J. Lipid Res. 20, 349–356 (1979).
Lobo, M. V. et al. Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids. J. Histochem. Cytochem. 49, 1253–1260 (2001).
Lynes, M., Narisawa, S., Millan, J. L. & Widmaier, E. P. Interactions between CD36 and global intestinal alkaline phosphatase in mouse small intestine and effects of high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R1738–R1747 (2011).
Nassir, F., Wilson, B., Han, X., Gross, R. W. & Abumrad, N. A. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J. Biol. Chem. 282, 19493–19501 (2007).
Drover, V. A. et al. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J. Clin. Invest. 115, 1290–1297 (2005).
Nauli, A. M. et al. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 131, 1197–1207 (2006).
Stahl, A. et al. Identification of the major intestinal fatty acid transport protein. Mol. Cell 4, 299–308 (1999).
Milger, K. et al. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J. Cell Sci. 119, 4678–4688 (2006).
Gimeno, R. E. et al. Targeted deletion of fatty acid transport protein-4 results in early embryonic lethality. J. Biol. Chem. 278, 49512–49516 (2003).
Moulson, C. L. et al. Cloning of wrinkle-free, a previously uncharacterized mouse mutation, reveals crucial roles for fatty acid transport protein 4 in skin and hair development. Proc. Natl Acad. Sci. USA 100, 5274–5279 (2003).
Herrmann, T. et al. Mice with targeted disruption of the fatty acid transport protein 4 (Fatp 4, Slc27a4) gene show features of lethal restrictive dermopathy. J. Cell Biol. 161, 1105–1115 (2003).
Moulson, C. L. et al. Keratinocyte-specific expression of fatty acid transport protein 4 rescues the wrinkle-free phenotype in Slc27a4/Fatp4 mutant mice. J. Biol. Chem. 282, 15912–15920 (2007).
Shim, J. et al. Fatty acid transport protein 4 is dispensable for intestinal lipid absorption in mice. J. Lipid Res. 50, 491–500 (2009).
Niot, I., Poirier, H., Tran, T. T. & Besnard, P. Intestinal absorption of long-chain fatty acids: evidence and uncertainties. Prog. Lipid Res. 48, 101–115 (2009).
Siddiqi, S., Sheth, A., Patel, F., Barnes, M. & Mansbach, C. M. 2nd Intestinal caveolin-1 is important for dietary fatty acid absorption. Biochim. Biophys. Acta 1831, 1311–1321 (2013).
Thumser, A. E. & Storch, J. Liver and intestinal fatty acid-binding proteins obtain fatty acids from phospholipid membranes by different mechanisms. J. Lipid Res. 41, 647–656 (2000).
Hsu, K. T. & Storch, J. Fatty acid transfer from liver and intestinal fatty acid-binding proteins to membranes occurs by different mechanisms. J. Biol. Chem. 271, 13317–13323 (1996).
Corsico, B., Cistola, D. P., Frieden, C. & Storch, J. The helical domain of intestinal fatty acid binding protein is critical for collisional transfer of fatty acids to phospholipid membranes. Proc. Natl Acad. Sci. USA 95, 12174–12178 (1998).
Storch, J. & Corsico, B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr. 28, 73–95 (2008).
Van Heek, M. et al. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J. Pharmacol. Exp. Ther. 283, 157–163 (1997).
Sylven, C. & Borgstrom, B. Absorption and lymphatic transport of cholesterol in the rat. J. Lipid Res. 9, 596–601 (1968).
Turley, S. D. & Dietschy, J. M. Sterol absorption by the small intestine. Curr. Opin. Lipidol. 14, 233–240 (2003).
Altmann, S. W. et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science 303, 1201–1204 (2004).
Jia, L., Betters, J. L. & Yu, L. Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport. Annu. Rev. Physiol. 73, 239–259 (2011).
Wang, L. J. et al. Molecular characterization of the NPC1L1 variants identified from cholesterol low absorbers. J. Biol. Chem. 286, 7397–7408 (2011).
Geach, T. Coronary heart disease: NPC1L1 mutations lower CHD risk. Nat. Rev. Cardiol. 12, 3 (2015).
Myocardial Infarction Genetics Consortium Investigators, et al. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N. Engl. J. Med. 371, 2072–2082 (2014).
Kern, F. Jr. Normal plasma cholesterol in an 88-year-old man who eats 25 eggs a day. Mechanisms of adaptation. N. Engl. J. Med. 324, 896–899 (1991).
Li, P. S. et al. The clathrin adaptor Numb regulates intestinal cholesterol absorption through dynamic interaction with NPC1L1. Nat. Med. 20, 80–86 (2014).
Nihei, W. et al. NPC1L1-dependent intestinal cholesterol absorption requires ganglioside GM3 in membrane microdomains. J. Lipid Res. 59, 2181–2187 (2018).
Ge, L. et al. Flotillins play an essential role in Niemann-Pick C1-like 1-mediated cholesterol uptake. Proc. Natl Acad. Sci. USA 108, 551–556 (2011).
Ge, L. et al. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 7, 508–519 (2008).
Johnson, T. A. & Pfeffer, S. R. Ezetimibe-sensitive cholesterol uptake by NPC1L1 protein does not require endocytosis. Mol. Biol. Cell 27, 1845–1852 (2016).
Bietrix, F. et al. Accelerated lipid absorption in mice overexpressing intestinal SR-BI. J. Biol. Chem. 281, 7214–7219 (2006).
Mardones, P. et al. Hepatic cholesterol and bile acid metabolism and intestinal cholesterol absorption in scavenger receptor class B type I-deficient mice. J. Lipid Res. 42, 170–180 (2001).
Hayashi, A. A. et al. Intestinal SR-BI is upregulated in insulin-resistant states and is associated with overproduction of intestinal apoB48-containing lipoproteins. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G326–G337 (2011).
Lino, M. et al. Intestinal scavenger receptor class B type I as a novel regulator of chylomicron production in healthy and diet-induced obese states. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G350–G359 (2015).
Morel, E. et al. Cholesterol trafficking and raft-like membrane domain composition mediate scavenger receptor class B type 1-dependent lipid sensing in intestinal epithelial cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1863, 199–211 (2018).
Reboul, E. et al. Scavenger receptor class B type I (SR-BI) is involved in vitamin E transport across the enterocyte. J. Biol. Chem. 281, 4739–4745 (2006).
Reboul, E. Vitamin E intestinal absorption: regulation of membrane transport across the enterocyte. IUBMB Life 71, 416–423 (2019).
During, A., Dawson, H. D. & Harrison, E. H. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J. Nutr. 135, 2305–2312 (2005).
Borel, P. et al. CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J. Nutr. 143, 448–456 (2013).
Weingartner, O. et al. Vascular effects of diet supplementation with plant sterols. J. Am. Coll. Cardiol. 51, 1553–1561 (2008).
Berge, K. E. et al. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science 290, 1771–1775 (2000).
Lutjohann, D., Bjorkhem, I., Beil, U. F. & von Bergmann, K. Sterol absorption and sterol balance in phytosterolemia evaluated by deuterium-labeled sterols: effect of sitostanol treatment. J. Lipid Res. 36, 1763–1773 (1995).
Bjorkhem, I. et al. Oxysterols in the circulation of patients with the Smith-Lemli-Opitz syndrome: abnormal levels of 24S- and 27-hydroxycholesterol. J. Lipid Res. 42, 366–371 (2001).
Bhattacharyya, A. K. & Connor, W. E. Beta-sitosterolemia and xanthomatosis. A newly described lipid storage disease in two sisters. J. Clin. Invest. 53, 1033–1043 (1974).
Miettinen, T. A. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: a case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur. J. Clin. Invest. 10, 27–35 (1980).
Hu, M., Yuen, Y. P., Kwok, J. S., Griffith, J. F. & Tomlinson, B. Potential effects of NPC1L1 polymorphisms in protecting against clinical disease in a chinese family with sitosterolaemia. J. Atheroscler. Thromb. 21, 989–995 (2014).
Plosch, T. et al. Sitosterolemia in ABC-transporter G5-deficient mice is aggravated on activation of the liver-X receptor. Gastroenterology 126, 290–300 (2004).
Yu, L. et al. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc. Natl Acad. Sci. USA 99, 16237–16242 (2002).
Wang, J. et al. Relative roles of ABCG5/ABCG8 in liver and intestine. J. Lipid Res. 56, 319–330 (2015).
Temel, R. E. & Brown, J. M. A new framework for reverse cholesterol transport: non-biliary contributions to reverse cholesterol transport. World J. Gastroenterol. 16, 5946–5952 (2010).
Temel, R. E. & Brown, J. M. A new model of reverse cholesterol transport: enTICEing strategies to stimulate intestinal cholesterol excretion. Trends Pharmacol. Sci. 36, 440–451 (2015).
Jakulj, L. et al. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 24, 783–794 (2016).
van der Veen, J. N. et al. Activation of the liver X receptor stimulates trans-intestinal excretion of plasma cholesterol. J. Biol. Chem. 284, 19211–19219 (2009).
Kruit, J. K. et al. Increased fecal neutral sterol loss upon liver X receptor activation is independent of biliary sterol secretion in mice. Gastroenterology 128, 147–156 (2005).
de Boer, J. F. et al. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology 152, 1126–1138.e6 (2017).
Vrins, C. L. et al. Peroxisome proliferator-activated receptor delta activation leads to increased transintestinal cholesterol efflux. J. Lipid Res. 50, 2046–2054 (2009).
Siddiqi, S. & Mansbach, C. M. 2nd Dietary and biliary phosphatidylcholine activates PKCζ in rat intestine. J. Lipid Res. 56, 859–870 (2015).
Kayden, H. J., Senior, J. R. & Mattson, F. H. The monoglyceride pathway of fat absorption in man. J. Clin. Invest. 46, 1695–1703 (1967).
Babayan, V. K. Medium chain triglycerides and structured lipids. Lipids 22, 417–420 (1987).
Yen, C. L. & Farese, R. V. Jr. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 278, 18532–18537 (2003).
Cheng, D. et al. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J. Biol. Chem. 278, 13611–13614 (2003).
Yue, Y. G. et al. The acyl coenzymeA:monoacylglycerol acyltransferase 3 (MGAT3) gene is a pseudogene in mice but encodes a functional enzyme in rats. Lipids 46, 513–520 (2011).
Nelson, D. W., Gao, Y., Yen, M. I. & Yen, C. L. Intestine-specific deletion of acyl-CoA:monoacylglycerol acyltransferase (MGAT) 2 protects mice from diet-induced obesity and glucose intolerance. J. Biol. Chem. 289, 17338–17349 (2014).
Gao, Y., Nelson, D. W., Banh, T., Yen, M. I. & Yen, C. L. E. Intestine-specific expression of MOGAT2 partially restores metabolic efficiency in Mogat2-deficient mice. J. Lipid Res. 54, 1644–1652 (2013).
Hall, A. M. et al. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. J. Lipid Res. 53, 990–999 (2012).
Cao, J., Cheng, L. & Shi, Y. Catalytic properties of MGAT3, a putative triacylgycerol synthase. J. Lipid Res. 48, 583–591 (2007).
Brandt, C., McFie, P. J. & Stone, S. J. Biochemical characterization of human acyl coenzyme A: 2-monoacylglycerol acyltransferase-3 (MGAT3). Biochem. Biophys. Res. Commun. 475, 264–270 (2016).
Smith, S. J. et al. Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat. Genet. 25, 87–90 (2000).
Chen, H. C., Ladha, Z., Smith, S. J. & Farese, R. V. Jr Analysis of energy expenditure at different ambient temperatures in mice lacking DGAT1. Am. J. Physiol. Endocrinol. Metab. 284, E213–E218 (2003).
Stone, S. J. et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J. Biol. Chem. 279, 11767–11776 (2004).
van Rijn, J. M. et al. Intestinal failure and aberrant lipid metabolism in patients with DGAT1 deficiency. Gastroenterology 155, 130–143.e15 (2018).
Haas, J. T. et al. DGAT1 mutation is linked to a congenital diarrheal disorder. J. Clin. Invest. 122, 4680–4684 (2012).
Dawson, P. A. & Rudel, L. L. Intestinal cholesterol absorption. Curr. Opin. Lipidol. 10, 315–320 (1999).
Nguyen, T. M., Sawyer, J. K., Kelley, K. L., Davis, M. A. & Rudel, L. L. Cholesterol esterification by ACAT2 is essential for efficient intestinal cholesterol absorption: evidence from thoracic lymph duct cannulation. J. Lipid Res. 53, 95–104 (2012).
Lee, O., Chang, C. C., Lee, W. & Chang, T. Y. Immunodepletion experiments suggest that acyl-coenzyme A:cholesterol acyltransferase-1 (ACAT-1) protein plays a major catalytic role in adult human liver, adrenal gland, macrophages, and kidney, but not in intestines. J. Lipid Res. 39, 1722–1727 (1998).
Meiner, V. et al. Tissue expression studies on the mouse acyl-CoA: cholesterol acyltransferase gene (Acact): findings supporting the existence of multiple cholesterol esterification enzymes in mice. J. Lipid Res. 38, 1928–1933 (1997).
Anderson, R. A. et al. Identification of a form of acyl-CoA:cholesterol acyltransferase specific to liver and intestine in nonhuman primates. J. Biol. Chem. 273, 26747–26754 (1998).
Cases, S. et al. ACAT-2, a second mammalian acyl-CoA:cholesterol acyltransferase. Its cloning, expression, and characterization. J. Biol. Chem. 273, 26755–26764 (1998).
Buhman, K. K. et al. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat. Med. 6, 1341–1347 (2000).
Repa, J. J., Buhman, K. K., Farese, R. V. Jr., Dietschy, J. M. & Turley, S. D. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology 40, 1088–1097 (2004).
Meiner, V. L. et al. Disruption of the acyl-CoA:cholesterol acyltransferase gene in mice: evidence suggesting multiple cholesterol esterification enzymes in mammals. Proc. Natl Acad. Sci. USA 93, 14041–14046 (1996).
O’Doherty, P. J., Kakis, G. & Kuksis, A. Role of luminal lecithin in intestinal fat absorption. Lipids 8, 249–255 (1973).
Rong, X. et al. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. eLife 4, e06557 (2015).
Li, Z. et al. Deficiency in lysophosphatidylcholine acyltransferase 3 reduces plasma levels of lipids by reducing lipid absorption in mice. Gastroenterology 149, 1519–1529 (2015).
Kabir, I. et al. Small intestine but not liver lysophosphatidylcholine acyltransferase 3 (Lpcat3) deficiency has a dominant effect on plasma lipid metabolism. J. Biol. Chem. 291, 7651–7660 (2016).
Demignot, S., Beilstein, F. & Morel, E. Triglyceride-rich lipoproteins and cytosolic lipid droplets in enterocytes: key players in intestinal physiology and metabolic disorders. Biochimie 96, 48–55 (2014).
Sabesin, S. M. & Frase, S. Electron microscopic studies of the assembly, intracellular transport, and secretion of chylomicrons by rat intestine. J. Lipid Res. 18, 496–511 (1977).
de Wit, N. J. et al. The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med. Genomics 1, 14 (2008).
Kondo, H. et al. Differential regulation of intestinal lipid metabolism-related genes in obesity-resistant A/J vs. obesity-prone C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 291, E1092–E1099 (2006).
Hayashi, H. et al. Fat feeding increases size, but not number, of chylomicrons produced by small intestine. Am. J. Physiol. 259, G709–G719 (1990).
Morel, E. et al. Lipid-dependent bidirectional traffic of apolipoprotein B in polarized enterocytes. Mol. Biol. Cell 15, 132–141 (2004).
Atzel, A. & Wetterau, J. R. Identification of two classes of lipid molecule binding sites on the microsomal triglyceride transfer protein. Biochemistry 33, 15382–15388 (1994).
Gordon, D. A., Jamil, H., Gregg, R. E., Olofsson, S. O. & Boren, J. Inhibition of the microsomal triglyceride transfer protein blocks the first step of apolipoprotein B lipoprotein assembly but not the addition of bulk core lipids in the second step. J. Biol. Chem. 271, 33047–33053 (1996).
Atzel, A. & Wetterau, J. R. Mechanism of microsomal triglyceride transfer protein catalyzed lipid transport. Biochemistry 32, 10444–10450 (1993).
Hussain, M. M., Bakillah, A., Nayak, N. & Shelness, G. S. Amino acids 430-570 in apolipoprotein B are critical for its binding to microsomal triglyceride transfer protein. J. Biol. Chem. 273, 25612–25615 (1998).
Jiang, Z. G., Liu, Y., Hussain, M. M., Atkinson, D. & McKnight, C. J. Reconstituting initial events during the assembly of apolipoprotein B-containing lipoproteins in a cell-free system. J. Mol. Biol. 383, 1181–1194 (2008).
Xie, Y. et al. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J. Biol. Chem. 281, 4075–4086 (2006).
Wetterau, J. R. et al. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258, 999–1001 (1992).
Ricci, B. et al. A 30-amino acid truncation of the microsomal triglyceride transfer protein large subunit disrupts its interaction with protein disulfide-isomerase and causes abetalipoproteinemia. J. Biol. Chem. 270, 14281–14285 (1995).
Muller, D. P. Vitamin E and neurological function. Mol. Nutr. Food Res. 54, 710–718 (2010).
Runge, P. et al. Oral vitamin E supplements can prevent the retinopathy of abetalipoproteinaemia. Br. J. Ophthalmol. 70, 166–173 (1986).
Muller, D. P., Lloyd, J. K. & Bird, A. C. Long-term management of abetalipoproteinaemia. Possible role for vitamin E. Arch. Dis. Child. 52, 209–214 (1977).
Hooper, A. J. et al. Postprandial lipoprotein metabolism in familial hypobetalipoproteinemia. J. Clin. Endocrinol. Metab. 92, 1474–1478 (2007).
Schonfeld, G., Lin, X. & Yue, P. Familial hypobetalipoproteinemia: genetics and metabolism. Cell Mol. Life Sci. 62, 1372–1378 (2005).
Lehner, R., Lian, J. & Quiroga, A. D. Lumenal lipid metabolism: implications for lipoprotein assembly. Arterioscler. Thromb. Vasc. Biol. 32, 1087–1093 (2012).
Yang, L. Y. & Kuksis, A. Apparent convergence (at 2-monoacylglycerol level) of phosphatidic acid and 2-monoacylglycerol pathways of synthesis of chylomicron triacylglycerols. J. Lipid Res. 32, 1173–1186 (1991).
Halpern, J., Tso, P. & Mansbach, C. M. 2nd Mechanism of lipid mobilization by the small intestine after transport blockade. J. Clin. Invest. 82, 74–81 (1988).
Nutting, D. F., Kumar, N. S., St Hilaire, R. J. & Mansbach, C. M. 2nd Nutrient absorption. Curr. Opin. Clin. Nutr. Metab. Care 2, 413–419 (1999).
Lu, S. et al. Overexpression of apolipoprotein A-IV enhances lipid secretion in IPEC-1 cells by increasing chylomicron size. J. Biol. Chem. 281, 3473–3483 (2006).
Kohan, A. B. et al. Apolipoprotein A-IV regulates chylomicron metabolism-mechanism and function. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G628–G636 (2012).
Kohan, A. B. et al. Is apolipoprotein A-IV rate limiting in the intestinal transport and absorption of triglyceride? Am. J. Physiol. Gastrointest. Liver Physiol. 304, G1128–G1135 (2013).
Black, D. D. Development and physiological regulation of intestinal lipid absorption. I. Development of intestinal lipid absorption: cellular events in chylomicron assembly and secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G519–524 (2007).
Ostos, M. A. et al. Antioxidative and antiatherosclerotic effects of human apolipoprotein A-IV in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 21, 1023–1028 (2001).
Qin, X., Swertfeger, D. K., Zheng, S., Hui, D. Y. & Tso,P. Apolipoprotein AIV: a potent endogenous inhibitor of lipid oxidation. Am. J. Physiol. 274, H1836–H1840 (1998).
Vowinkel, T. et al. Apolipoprotein A-IV inhibits experimental colitis. J. Clin. Invest. 114, 260–269 (2004).
Fujimoto, K., Cardelli, J. A. & Tso, P. Increased apolipoprotein A-IV in rat mesenteric lymph after lipid meal acts as a physiological signal for satiation. Am. J. Physiol. 262, G1002–G1006 (1992).
Fujimoto, K., Fukagawa, K., Sakata, T. & Tso, P. Suppression of food intake by apolipoprotein A-IV is mediated through the central nervous system in rats. J. Clin. Invest. 91, 1830–1833 (1993).
Lo, C. C. et al. Apolipoprotein AIV requires cholecystokinin and vagal nerves to suppress food intake. Endocrinology 153, 5857–5865 (2012).
Li, X., Wang, F., Xu, M., Howles, P. & Tso, P. ApoA-IV improves insulin sensitivity and glucose uptake in mouse adipocytes via PI3K-Akt signaling. Sci. Rep. 7, 41289 (2017).
Wang, F. et al. Apolipoprotein A-IV improves glucose homeostasis by enhancing insulin secretion. Proc. Natl Acad. Sci. USA 109, 9641–9646 (2012).
Li, X. et al. Apolipoprotein A-IV reduces hepatic gluconeogenesis through nuclear receptor NR1D1. J. Biol. Chem. 289, 2396–2404 (2014).
Xu, X. R. et al. Apolipoprotein A-IV binds αIIbβ3 integrin and inhibits thrombosis. Nat. Commun. 9, 3608 (2018).
Wong, W. M. et al. Apolipoprotein AIV gene variant S347 is associated with increased risk of coronary heart disease and lower plasma apolipoprotein AIV levels. Circ. Res. 92, 969–975 (2003).
Kronenberg, F. et al. Low apolipoprotein A-IV plasma concentrations in men with coronary artery disease. J. Am. Coll. Cardiol. 36, 751–757 (2000).
Rao, R. et al. Circulating apolipoprotein A-IV presurgical levels are associated with improvement in insulin sensitivity after Roux-en-Y gastric bypass surgery. Surg. Obes. Relat. Dis. 13, 468–473 (2017).
Mansbach, C. M. 2nd & Nevin, P. Intracellular movement of triacylglycerols in the intestine. J. Lipid Res. 39, 963–968 (1998).
Siddiqi, S. et al. A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J. Lipid Res. 51, 1918–1928 (2010).
Siddiqi, S. A., Mahan, J., Siddiqi, S., Gorelick, F. S. & Mansbach, C. M. 2nd. Vesicle-associated membrane protein 7 is expressed in intestinal ER. J. Cell Sci. 119, 943–950 (2006).
Sane, A. T. et al. Understanding chylomicron retention disease through Sar1b Gtpase gene disruption: insight from cell culture. Arterioscler. Thromb. Vasc. Biol. 37, 2243–2251 (2017).
Charcosset, M. et al. Anderson or chylomicron retention disease: molecular impact of five mutations in the SAR1B gene on the structure and the functionality of Sar1b protein. Mol. Genet. Metab. 93, 74–84 (2008).
Siddiqi, S., Siddiqi, S. A. & Mansbach, C. M. 2nd. Sec24C is required for docking the prechylomicron transport vesicle with the Golgi. J. Lipid Res. 51, 1093–1100 (2010).
Siddiqi, S. A. et al. The identification of a novel endoplasmic reticulum to Golgi SNARE complex used by the prechylomicron transport vesicle. J. Biol. Chem. 281, 20974–20982 (2006).
Katz, L., Hanson, P. I., Heuser, J. E. & Brennwald, P. Genetic and morphological analyses reveal a critical interaction between the C-termini of two SNARE proteins and a parallel four helical arrangement for the exocytic SNARE complex. EMBO J. 17, 6200–6209 (1998).
Siddiqi, S. A., Gorelick, F. S., Mahan, J. T. & Mansbach, C. M. 2nd. COPII proteins are required for Golgi fusion but not for endoplasmic reticulum budding of the pre-chylomicron transport vesicle. J. Cell Sci. 116, 415–427 (2003).
Berriot-Varoqueaux, N. et al. Apolipoprotein B48 glycosylation in abetalipoproteinemia and Anderson’s disease. Gastroenterology 121, 1101–1108 (2001).
Levy, E., Poinsot, P. & Spahis, S. Chylomicron retention disease: genetics, biochemistry, and clinical spectrum. Curr. Opin. Lipidol. 30, 134–139 (2019).
Tso, P., Balint, J. A. & Rodgers, J. B. Effect of hydrophobic surfactant (Pluronic L-81) on lymphatic lipid transport in the rat. Am. J. Physiol. 239, G348–G353 (1980).
Kvietys, P. R., Specian, R. D., Grisham, M. B. & Tso, P. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. Am. J. Physiol. 261, G384–G391 (1991).
Jattan, J. et al. Using primary murine intestinal enteroids to study dietary TAG absorption, lipoprotein synthesis, and the role of apoC-III in the intestine. J. Lipid Res. 58, 853–865 (2017).
Wang, F. et al. Overexpression of apolipoprotein C-III decreases secretion of dietary triglyceride into lymph. Physiol. Rep. 2, e00247 (2014).
Windler, E., Chao, Y. & Havel, R. J. Determinants of hepatic uptake of triglyceride-rich lipoproteins and their remnants in the rat. J. Biol. Chem. 255, 5475–5480 (1980).
Tso, P. & Gollamudi, S. R. Pluronic L-81: a potent inhibitor of the transport of intestinal chylomicrons. Am. J. Physiol. 247, G32–G36 (1984).
Ockner, R. K., Hughes, F. B. & Isselbacher, K. J. Very low density lipoproteins in intestinal lymph: role in triglyceride and cholesterol transport during fat absorption. J. Clin. Invest. 48, 2367–2373 (1969).
Mahley, R. W. et al. Lipoproteins associated with the Golgi apparatus isolated from epithelial cells of rat small intestine. Lab. Invest. 25, 435–444 (1971).
Nutting, D., Hall, J., Barrowman, J. A. & Tso, P. Further studies on the mechanism of inhibition of intestinal chylomicron transport by Pluronic L-81. Biochim. Biophys. Acta 1004, 357–362 (1989).
Glickman, R. M. & Green, P. H. The intestine as a source of apolipoprotein A1. Proc. Natl Acad. Sci. USA 74, 2569–2573 (1977).
Jonas, A. Lecithin cholesterol acyltransferase. Biochim. Biophys. Acta 1529, 245–256 (2000).
Connelly, M. A. & Williams, D. L. SR-BI and HDL cholesteryl ester metabolism. Endocr. Res. 30, 697–703 (2004).
Brunham, L. R. et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116, 1052–1062 (2006).
Repa, J. J. et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289, 1524–1529 (2000).
Iqbal, J., Boutjdir, M., Rudel, L. L. & Hussain, M. M. Intestine-specific MTP and global ACAT2 deficiency lowers acute cholesterol absorption with chylomicrons and HDLs. J. Lipid Res. 55, 2261–2275 (2014).
Rashid, S., Watanabe, T., Sakaue, T. & Lewis, G. F. Mechanisms of HDL lowering in insulin resistant, hypertriglyceridemic states: the combined effect of HDL triglyceride enrichment and elevated hepatic lipase activity. Clin. Biochem. 36, 421–429 (2003).
Patsch, J. R., Gotto, A. M. Jr., Olivercrona, T. & Eisenberg, S. Formation of high density lipoprotein2-like particles during lipolysis of very low density lipoproteins in vitro. Proc. Natl Acad. Sci. USA 75, 4519–4523 (1978).
Iqbal, J., Parks, J. S. & Hussain, M. M. Lipid absorption defects in intestine-specific microsomal triglyceride transfer protein and ATP-binding cassette transporter A1-deficient mice. J. Biol. Chem. 288, 30432–30444 (2013).
Zhu, J., Lee, B., Buhman, K. K. & Cheng, J. X. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-stokes Raman scattering imaging. J. Lipid Res. 50, 1080–1089 (2009).
Kassan, A. et al. Acyl-CoA synthetase 3 promotes lipid droplet biogenesis in ER microdomains. J. Cell Biol. 203, 985–1001 (2013).
Wilfling, F., Haas, J. T., Walther, T. C. & Farese, R. V. Jr. Lipid droplet biogenesis. Curr. Opin. Cell Biol. 29, 39–45 (2014).
Hung, Y. H., Carreiro, A. L. & Buhman, K. K. Dgat1 and Dgat2 regulate enterocyte triacylglycerol distribution and alter proteins associated with cytoplasmic lipid droplets in response to dietary fat. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1862, 600–614 (2017).
D’Aquila, T. et al. Characterization of the proteome of cytoplasmic lipid droplets in mouse enterocytes after a dietary fat challenge. PLOS ONE 10, e0126823 (2015).
Lee, B., Zhu, J., Wolins, N. E., Cheng, J. X. & Buhman, K. K. Differential association of adipophilin and TIP47 proteins with cytoplasmic lipid droplets in mouse enterocytes during dietary fat absorption. Biochim. Biophys. Acta 1791, 1173–1180 (2009).
Itabe, H., Yamaguchi, T., Nimura, S. & Sasabe, N. Perilipins: a diversity of intracellular lipid droplet proteins. Lipids Health Dis. 16, 83 (2017).
Bouchoux, J. et al. The proteome of cytosolic lipid droplets isolated from differentiated Caco-2/TC7 enterocytes reveals cell-specific characteristics. Biol. Cell 103, 499–517 (2011).
Frank, D. N. et al. Perilipin-2 modulates lipid absorption and microbiome responses in the mouse intestine. PLOS ONE 10, e0131944 (2015).
Beilstein, F., Carriere, V., Leturque, A. & Demignot, S. Characteristics and functions of lipid droplets and associated proteins in enterocytes. Exp. Cell Res. 340, 172–179 (2016).
Obrowsky, S. et al. Adipose triglyceride lipase is a TG hydrolase of the small intestine and regulates intestinal PPARα signaling. J. Lipid Res. 54, 425–435 (2013).
Khaldoun, S. A. et al. Autophagosomes contribute to intracellular lipid distribution in enterocytes. Mol. Biol. Cell 25, 118–132 (2014).
Du, H. et al. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J. Lipid Res. 42, 489–500 (2001).
Porto, A. F. Lysosomal acid lipase deficiency: diagnosis and treatment of Wolman and cholesteryl ester storage diseases. Pediatr. Endocrinol. Rev. 12 (Suppl. 1), 125–132 (2014).
Soayfane, Z. et al. Exposure to dietary lipid leads to rapid production of cytosolic lipid droplets near the brush border membrane. Nutr. Metab. 13, 48 (2016).
Accioly, M. T. et al. Lipid bodies are reservoirs of cyclooxygenase-2 and sites of prostaglandin-E2 synthesis in colon cancer cells. Cancer Res. 68, 1732–1740 (2008).
Moreira, L. S. et al. Cytosolic phospholipase A2-driven PGE2 synthesis within unsaturated fatty acids-induced lipid bodies of epithelial cells. Biochim. Biophys. Acta 1791, 156–165 (2009).
Storch, J., Zhou, Y. X. & Lagakos, W. S. Metabolism of apical versus basolateral sn-2-monoacylglycerol and fatty acids in rodent small intestine. J. Lipid Res. 49, 1762–1769 (2008).
Mansbach, C. M. 2nd & Dowell, R. F. Uptake and metabolism of circulating fatty acids by rat intestine. Am. J. Physiol. 263, G927–G933 (1992).
Lagakos, W. S. et al. Different functions of intestinal and liver-type fatty acid-binding proteins in intestine and in whole body energy homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G803–G814 (2011).
Thomson, A. B., Keelan, M., Clandinin, M. T. & Walker, K. Dietary fat selectively alters transport properties of rat jejunum. J. Clin. Invest. 77, 279–288 (1986).
Sukhotnik, I. et al. Effect of a high fat diet on lipid absorption and fatty acid transport in a rat model of short bowel syndrome. Pediatr. Surg. Int. 19, 385–390 (2003).
Uchida, A., Slipchenko, M. N., Cheng, J. X. & Buhman, K. K. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, alters triglyceride metabolism in enterocytes of mice. Biochim. Biophys. Acta 1811, 170–176 (2011).
Berger, J. & Wagner, J. A. Physiological and therapeutic roles of peroxisome proliferator-activated receptors. Diabetes Technol. Ther. 4, 163–174 (2002).
Mochizuki, K., Suruga, K., Kitagawa, M., Takase, S. & Goda, T. Modulation of the expression of peroxisome proliferator-activated receptor-dependent genes through disproportional expression of two subtypes in the small intestine. Arch. Biochem. Biophys. 389, 41–48 (2001).
Karimian Azari, E., Leitner, C., Jaggi, T., Langhans, W. & Mansouri, A. Possible role of intestinal fatty acid oxidation in the eating-inhibitory effect of the PPAR-α agonist Wy-14643 in high-fat diet fed rats. PLOS ONE 8, e74869 (2013).
Hooper, L. V. & Gordon, J. I. Commensal host-bacterial relationships in the gut. Science 292, 1115–1118 (2001).
El Aidy, S. et al. The gut microbiota elicits a profound metabolic reorientation in the mouse jejunal mucosa during conventionalisation. Gut 62, 1306–1314 (2013).
Donaldson, G. P., Lee, S. M. & Mazmanian, S. K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 14, 20–32 (2016).
Backhed, F., Manchester, J. K., Semenkovich, C. F. & Gordon, J. I. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl Acad. Sci. USA 104, 979–984 (2007).
Rabot, S. et al. Germ-free C57BL/6J mice are resistant to high-fat-diet-induced insulin resistance and have altered cholesterol metabolism. FASEB J. 24, 4948–4959 (2010).
Martinez-Guryn, K. et al. Small intestine microbiota regulate host digestive and absorptive adaptive responses to dietary lipids. Cell Host Microbe 23, 458–469.e5 (2018).
Sato, H. et al. Antibiotics suppress activation of intestinal mucosal mast cells and reduce dietary lipid absorption in sprague-dawley rats. Gastroenterology 151, 923–932 (2016).
Ji, Y. et al. Activation of rat intestinal mucosal mast cells by fat absorption. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1292–G1300 (2012).
Scudamore, C. L., Jepson, M. A., Hirst, B. H. & Miller, H. R. The rat mucosal mast cell chymase, RMCP-II, alters epithelial cell monolayer permeability in association with altered distribution of the tight junction proteins ZO-1 and occludin. Eur. J. Cell Biol. 75, 321–330 (1998).
Heck, A. M., Yanovski, J. A. & Calis, K. A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy 20, 270–279 (2000).
Hofmann, A. F. & Poley, J. R. Role of bile acid malabsorption in pathogenesis of diarrhea and steatorrhea in patients with ileal resection. I. Response to cholestyramine or replacement of dietary long chain triglyceride by medium chain triglyceride. Gastroenterology 62, 918–934 (1972).
Parkinson, T. M., Gundersen, K. & Nelson, N. A. Effects of colestipol (U-26,597A), a new bile acid sequestrant, on serum lipids in experimental animals and man. Atherosclerosis 11, 531–537 (1970).
Bilheimer, D. W., Grundy, S. M., Brown, M. S. & Goldstein, J. L. Mevinolin and colestipol stimulate receptor-mediated clearance of low density lipoprotein from plasma in familial hypercholesterolemia heterozygotes. Proc. Natl Acad. Sci. USA 80, 4124–4128 (1983).
Davidson, M. H. et al. Colesevelam hydrochloride (cholestagel): a new, potent bile acid sequestrant associated with a low incidence of gastrointestinal side effects. Arch. Intern. Med. 159, 1893–1900 (1999).
Okuma, C. et al. JTP-103237, a novel monoacylglycerol acyltransferase inhibitor, modulates fat absorption and prevents diet-induced obesity. Eur. J. Pharmacol. 758, 72–81 (2015).
Dow, R. L. et al. Discovery of PF-04620110, a potent, selective, and orally bioavailable inhibitor of DGAT-1. ACS Med. Chem. Lett. 2, 407–412 (2011).
Dow, R. L. et al. Defining the key pharmacophore elements of PF-04620110: discovery of a potent, orally-active, neutral DGAT-1 inhibitor. Bioorg. Med. Chem. 21, 5081–5097 (2013).
Rizzo, M. Lomitapide, a microsomal triglyceride transfer protein inhibitor for the treatment of hypercholesterolemia. IDrugs 13, 103–111 (2010).
Aggarwal, D. et al. JTT-130, a microsomal triglyceride transfer protein (MTP) inhibitor lowers plasma triglycerides and LDL cholesterol concentrations without increasing hepatic triglycerides in guinea pigs. BMC Cardiovasc. Disord. 5, 30 (2005).
Hata, T. et al. JTT-130, a novel intestine-specific inhibitor of microsomal triglyceride transfer protein, suppresses food intake and gastric emptying with the elevation of plasma peptide YY and glucagon-like peptide-1 in a dietary fat-dependent manner. J. Pharmacol. Exp. Ther. 336, 850–856 (2011).
Acknowledgements
The authors are grateful for the support of National Institutes of Health Grants DK 103557, DK 119135, and DK 59630 (P.T.) and HD22551 (D.D.B.). The editorial assistance of A. Preston is greatly appreciated.
Author information
Authors and Affiliations
Contributions
C.-W.K. and D.D.B. researched data for the article. All authors contributed equally to discussion of content, writing and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Gastroenterology & Hepatology thanks G. Lewis, M.M. Hussain and the other, anonymous, reviewer for their contribution to the peer review of this work.
Rights and permissions
About this article
Cite this article
Ko, CW., Qu, J., Black, D.D. et al. Regulation of intestinal lipid metabolism: current concepts and relevance to disease. Nat Rev Gastroenterol Hepatol 17, 169–183 (2020). https://doi.org/10.1038/s41575-019-0250-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-019-0250-7
This article is cited by
-
Integrated meta-omics reveals the regulatory landscape involved in lipid metabolism between pig breeds
Microbiome (2024)
-
Mitochondrial dysfunction abrogates dietary lipid processing in enterocytes
Nature (2024)
-
Epithelial zonation along the mouse and human small intestine defines five discrete metabolic domains
Nature Cell Biology (2024)
-
BMAL1 deletion protects against obesity and non-alcoholic fatty liver disease induced by a high-fat diet
International Journal of Obesity (2024)
-
Metabolomics and lipidomics signature in celiac disease: a narrative review
Clinical and Experimental Medicine (2024)