Abstract
The cochlea uses two types of mechanosensory cell to detect sounds. A single row of inner hair cells (IHCs) synapse onto neurons to transmit sensory information to the brain, and three rows of outer hair cells (OHCs) selectively amplify auditory inputs1. So far, two transcription factors have been implicated in the specific differentiation of OHCs, whereas, to our knowledge, none has been identified in the differentiation of IHCs2,3,4. One such transcription factor for OHCs, INSM1, acts during a crucial embryonic period to consolidate the OHC fate, preventing OHCs from transdifferentiating into IHCs2. In the absence of INSM1, embryonic OHCs misexpress a core set of IHC-specific genes, which we predict are involved in IHC differentiation. Here we find that one of these genes, Tbx2, is a master regulator of IHC versus OHC differentiation in mice. Ablation of Tbx2 in embryonic IHCs results in their development as OHCs, expressing early OHC markers such as Insm1 and eventually becoming completely mature OHCs in the position of IHCs. Furthermore, Tbx2 is epistatic to Insm1: in the absence of both genes, cochleae generate only OHCs, which suggests that TBX2 is necessary for the abnormal transdifferentiation of INSM1-deficient OHCs into IHCs, as well as for normal IHC differentiation. Ablation of Tbx2 in postnatal, largely differentiated IHCs makes them transdifferentiate directly into OHCs, replacing IHC features with those of mature and not embryonic OHCs. Finally, ectopic expression of Tbx2 in OHCs results in their transdifferentiation into IHCs. Hence, Tbx2 is both necessary and sufficient to make IHCs distinct from OHCs and maintain this difference throughout development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information. Source data are provided with this paper.
References
Dallos, P. In Proceedings of the 9th International Symposium on Hearing (eds Cazals, Y. et al.) 3–17 (Elsevier, 1992).
Wiwatpanit, T. et al. Trans-differentiation of outer hair cells into inner hair cells in the absence of INSM1. Nature 563, 691–695 (2018).
Chessum, L. et al. Helios is a key transcriptional regulator of outer hair cell maturation. Nature 563, 696–700 (2018).
Lorenzen, S. M., Duggan, A., Osipovich, A. B., Magnuson, M. A. & García-Añoveros, J. Insm1 promotes neurogenic proliferation in delaminated otic progenitors. Mech. Dev. 138, 233–245 (2015).
Kaiser, M. et al. Regulation of otocyst patterning by Tbx2 and Tbx3 is required for inner ear morphogenesis in the mouse. Development 148, dev195651 (2021).
Chen, Y. et al. The transcription factor TBX2 regulates melanogenesis in melanocytes by repressing Oca2. Mol. Cell. Biochem. 415, 103–109 (2016).
Aydoğdu, N. et al. TBX2 and TBX3 act downstream of canonical WNT signaling in patterning and differentiation of the mouse ureteric mesenchyme. Development 145, dev171827 (2018).
Wojahn, I., Lüdtke, T. H., Christoffels, V. M., Trowe, M.-O. & Kispert, A. TBX2-positive cells represent a multi-potent mesenchymal progenitor pool in the developing lung. Respir. Res. 20, 292 (2019).
Wakker, V. et al. Generation of mice with a conditional null allele for Tbx2. Genesis 48, 195–199 (2010).
Yang, H., Xie, X., Deng, M., Chen, X. & Gan, L. Generation and characterization of Atoh1-Cre knock-in mouse line. Genesis 48, 407–413 (2010).
Yang, H. et al. Gfi1-Cre knock-in mouse line: a tool for inner ear hair cell-specific gene deletion. Genesis 48, 400–406 (2010).
Sekerková, G., Richter, C.-P. & Bartles, J. R. Roles of the espin actin-bundling proteins in the morphogenesis and stabilization of hair cell stereocilia revealed in CBA/CaJ congenic jerker mice. PLoS Genet. 7, e1002032 (2011).
Nielsen, D. W. & Slepecky, N. Stereocilia. in Neurobiology of Hearing: The Cochlea (eds Altschuler, R. A. et al.) 23–46 (Raven Press, 1986).
Ratzan, E. M., Moon, A. M. & Deans, M. R. Fgf8 genetic labeling reveals the early specification of vestibular hair cell type in mouse utricle. Development 147, dev192849 (2020).
Webber, J. L. et al. Axodendritic versus axosomatic cochlear efferent termination is determined by afferent type in a hierarchical logic of circuit formation. Sci. Adv. 7, eabd8637 (2021).
Dabdoub, A. et al. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl Acad. Sci. USA 105, 18396–18401 (2008).
Kempfle, J. S., Turban, J. L. & Edge, A. S. B. Sox2 in the differentiation of cochlear progenitor cells. Sci. Rep. 6, 23293 (2016).
Cox, B. C. et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 141, 816–829 (2014).
Hu, L. et al. Diphtheria toxin-induced cell death triggers Wnt-dependent hair cell regeneration in neonatal mice. J. Neurosci. 36, 9479–9489 (2016).
Bramhall, N. F., Shi, F., Arnold, K., Hochedlinger, K. & Edge, A. S. B. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Rep. 2, 311–322 (2014).
Liu, H. et al. Cell-specific transcriptome analysis shows that adult pillar and Deiters’ cells express genes encoding machinery for specializations of cochlear hair cells. Front. Mol. Neurosci. 11, 356 (2018).
Liu, H. et al. Characterization of transcriptomes of cochlear inner and outer hair cells. J. Neurosci. 34, 11085–11095 (2014).
Jia, S., Yang, S., Guo, W. & He, D. Z. Z. Fate of mammalian cochlear hair cells and stereocilia after loss of the stereocilia. J. Neurosci. 29, 15277–15285 (2009).
Wang, Y., Hirose, K. & Liberman, M. C. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J. Assoc. Res. Otolaryngol. 3, 248–268 (2002).
Landegger, L. D. et al. A synthetic AAV vector enables safe and efficient gene transfer to the mammalian inner ear. Nat. Biotechnol. 35, 280–284 (2017).
Coffin, A., Kelley, M., Manley, G. A. & Popper, A. N. Evolution of sensory hair cells. in Evolution of the Vertebrate Auditory System, Vol. 22 (eds Manley, G. A. et al.) 55–94 (Springer, 2004).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133–140 (2010).
Haque, K. D., Pandey, A. K., Kelley, M. W. & Puligilla, C. Culture of embryonic mouse cochlear explants and gene transfer by electroporation. J. Vis. Exp. 12, 52260 (2015).
Pearce, M., Richter, C.-P. & Cheatham, M. A. A reconsideration of sound calibration in the mouse. J. Neurosci. Meth. 106, 57–67 (2001).
Neely, S. T. & Liu, Z. EMAV: Otoacoustic Emission Averager. Technical memo no. 17 (Boy’s Town National Research Hospital, 1994).
Santos-Sacchi, J., Kakehata, S. & Takahashi, S. Effects of membrane potential on the voltage dependence of motility‐related charge in outer hair cells of the guinea‐pig. J. Physiol. 510, 225–235 (1998).
Homma, K. & Dallos, P. Evidence that prestin has at least two voltage-dependent steps. J. Biol. Chem. 286, 2297–2307 (2011).
Acknowledgements
NUcore facilities used were the Transgenic and Targeted Mutagenesis Laboratory and the Center for Advanced Microscopy (partially supported by P30-CA060553 to the Robert H. Lurie Comprehensive Cancer Center). This study was supported by National Institutes of Health grants R01-DC015903, R01-DC019834 and R01-DC017482. The expertise of J.H. Siegel regarding hearing tests is appreciated.
Author information
Authors and Affiliations
Contributions
J.G.-A. and A.D. conceived the project and planed experiments. J.G.-A., J.C.C., A.D. and C.Z.F. performed and analysed experiments in mouse genetics. A.D. performed in situ hybridization. J.C.C., A.D., C.Z.F. and I.G.G. conducted immunohistochemistry. J.G.-A., J.C.C., C.Z.F. and I.G.G. performed confocal image collection and analysis. A.D. conducted light microscopy and quantification. I.G.G. conducted nuclear size determination and Imaris analysis. Y.Z. and M.A.C. conducted hearing tests. K.H. performed cellular electrophysiology. C.Z.F. and Y.Z. performed AAV-mediated expression in organotypic explants. J.G.-A. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Alan Cheng, Oliver Hobert and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Expression of Tbx2 mRNA in cochlea.
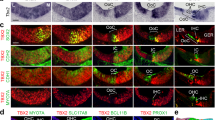
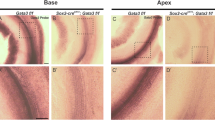
(a,d) In situ hybridizations in sections of neonatal (P0 and P1) cochleae from control mice (Insm1F/F) reveal Tbx2 expression in most cells lining the scala media including IHCs, Inner Phalangeal Cells (IPhC), Inner Border Cells (IBC), Greater Epithelial Ridge (GER), interdental cells of the Spiral Limbus (SL), Reissner’s Membrane (RM), some cells of the Stria Vascularis (SV), Spiral Prominence (SP), and Claudius’ Cells (CCs); but little or no expression (above background levels) of Tbx2 in OHCs or other cells of the outer compartment, namely Hensen’s Cells (HeCs), Deiters’ Cells (DCs), and Outer and Inner Pillar cells (OPC and IPC). (b,c,e) In situ hybridizations in sections of developing Insm1 mutant cochleae (Atoh1Cre/+; Insm1F/F in b and c; TgPax2Cre/+; Insm1F/F in e) reveal that Tbx2 is expressed in about half of the OHCs, presumably those transdifferentiating into IHCs. In controls, none of the OHCs (0/117 from N=4 Insm1F/F mice at E16.5, P0 and P1) expressed Tbx2, whereas in Insm1 mutants, in which nearly half of the OHCs transdifferentiate into IHCs (46.0±5.6%)2, an equivalent fraction of hair cells in the position of the OHCs expressed Tbx2 (46.6%; 62/133 cells from N=4 TgPax2Cre/+; Insm1F/F or Atoh1Cre/+; Insm1F/F mice at E16.5, P0 and P1). (f-h) Double in situ hybridizations in sections of neonatal (P0) cochleae for detection of Tbx2 (magenta) and Vglut3 (blue), the earliest marker of functional, mature IHCs. (f) In Insm1F/F controls, only IHCs express high levels of Vglut3 (a few dots label OHCs, perhaps representing a much lower level of expression). (g,h) In TgPax2Cre/+; Insm1F/F cochleae, only OHCs misexpressing Tbx2 (50/114 at P0, 14/30 at P2 and 9/21 at P4) also misexpressed high levels (those characteristic of IHCs) of Vglut3, whereas none of the OHCs misexpressed either gene alone (at IHC-like levels). Hence, early expression of Tbx2 in OHCs lacking INSM1 appears to correlate perfectly with their transdifferentiating into IHCs. Images shown are examples of results obtained in three separate tissue samples (n=3 biologically independent samples). Scale bar of 20 µm in (a) applies to all other panels.
Extended Data Fig. 2 Nuclear volumes of hair cells in the inner or outer compartment of controls and in various Tbx2 conditional KOs.
Blue dots represent nuclear volumes of hair cells from the inner compartment (I in the column labels), whether IHCs (controls) or ic-HCs (mutants). Red dots represent nuclear volumes of hair cells from the outer compartment (OHCs; O in the column labels). Sample size (n) is indicated in each column. The time of tamoxifen administration to Fgf8CreER; Tbx2F/F pups is indicated in the column labels as TAM@P0 to P9. (a,b) While in controls the nuclei of IHCs are statistically larger than those of OHCs, in the mutants (all Tbx2 cKOs as well as the double cKO of Tbx2 and Insm1) the nuclei of the inner compartment hair cells (ic-HCs) are statistically smaller than those of control IHCs (b) and indistinguishable from those of OHCs (a). Error bars indicate SD and column tops the averages. Statistical comparisons were performed by one-way ANOVA with a Bonferroni post test. **** indicates P<0.0001, while ns (not significant) indicates P>0.05 (for panel a, from left to right, P=0.0792 for Atoh1CreTbx2F/F; P>0.9999 for Gfi1CreTbx2F/F; P=0.1285 for Atoh1CreGfi1CreTbx2F/F; P>0.9999 for Fgf8CreERTbx2F/F + Tamoxifen at P0, P7 and P9)
Extended Data Fig. 3 Non-linear capacitance parameters evince electromotility of ic-OHCs.
(a,a’) Hair cells in the position of IHCs (ic-OHCs) that lacked TBX2 (from Fgf8CreER; Tbx2F/F; R26LSL-tdTomato/+ treated with tamoxifen at birth) were identified by their red fluorescence (tdTomato+; a’), whereas control IHCs and OHCs were obtained from equally treated littermates that lacked Fgf8CreER (Tbx2F/F; R26LSL-tdTomato/+), so that their IHCs did not transdifferentiate into ic-OHCs, expressing IHC markers like Calb2 and not tdTomato (a). These control IHC and OHC were distinguished as before15. Images shown are examples of results obtained in at least three separate tissue samples (n=3 biologically independent samples). (b) Summary of the non-linear capacitance (NLC) parameters of ic-OHCs lacking TBX2 and control OHCs (including those examined in Fig. 1s, t). Like control OHCs (n=9), all examined ic-OHCs (n=10) were electromotile. Samples of each cell type were obtained from ≥ 3 separate animals. Horizontal bars indicate the means and the standard deviations. Statistical comparisons were performed by unpaired t-tests (two-tailed). “ns” indicates not significant (p > 0.05)
Extended Data Fig. 4 Cochlear hair cells transdifferentiating postnatally transiently co-express markers of mature IHCs (VGLUT3) and OHCs (Prestin and PMCA2), but not of nascent hair cells (SOX2) nor of differentiating OHCs (Insm1 and Bcl11b).
Fgf8CreER; Tbx2F/F; R26LSL-tdTomato/+ newborn (P0) pups were treated with tamoxifen at P0 and collected for examination at each subsequent day from P1 to P8. (a-c) Immunohistochemistry at P5 reveals that hair cells in the position of the IHCs express the IHC functional marker VGLUT3 (and not the OHC developmental market BCL11b) (a; confocal optical section), but also the OHC functional markers Prestin (b; confocal optical section) and PMCA2 (c; confocal projection). In (c), former IHCs are identified by the expression of TdTomato. Hence, at this time transdifferentiating hair cells display features of both IHCs and OHCs. (d) Immunohistochemistry at P1, displayed as a confocal radial optical section, reveals that cells transdifferentiating from IHCs to OHCs (at this point ambiguously termed ic-HCs; TdTomato+ in the lower panel) do not express SOX2, a nuclear marker of both supporting cells and nascent hair cells, and are beginning to express PMCA2 in their stereocilia (bottom panel). For a better visualization of SOX2, the top panel displays its immunoreactivity only with the nuclear counterstain DAPI. (e,f) In situ hybridization combined with immunohistochemistry (for Myosin VIIa, to label IHCs and OHCs) on cryostat sections from P1 cochleae reveal that ic-HCs transdifferentiating from IHCs to ic-OHCs do not express Bcl11b (e) or Insm1 (f) mRNAs, which are expressed by OHCs during their early (embryonic to postnatal) differentiation. Images were taken at apical, middle and basal cochlear positions at P1 and subsequent postnatal days. Images shown are examples of results obtained in at least three separate tissue samples (n=3 biologically independent samples). All scale bars are 20 µm. (g) Schematic summary of all the stainings performed on mice at P1 to P8 in which Tbx2 ablation had been induced by tamoxifen administration at P0. Blue shading denotes the period (P1 to P7) during which transdifferentiating ic-HCs co-express VGLUT3 with OHC markers PMCA and/or Prestin. At no point from P1 to P8 do ic-HCs express the nascent HC marker SOX2 or the OHC differentiation markers Bcl11b (mRNA or protein) or Insm1. Expression levels are subjectively categorized as strong (+++) to undetectable above background (-). Expression in ic-HCs (red font) is provided by comparison to expression in the following control cells (blue font) in the same tissues or stages: Wild type (WT) IHCs for VGLUT3; adjacent OHCs for Prestin, PMCA2, Bcl11b and Insm1; and Supporting Cells (SC) for SOX2. Supporting and other cells labeled in (d) as expressing SOX2 are epithelial cells of Kölliker’s Organ (KO), Inner Border (IBC), Inner Phalangeal (IPhC), Inner Pillar (IPC), Outer Pillar (OPC), Deiters' (DC) and Hensen’s (HeC) cells.
Extended Data Fig. 5 Schematic illustration of the various forms of transdifferentiation between IHCs and OHCs.
IHCs and OHCs are depicted from their onset after proliferation of progenitors has ceased (nascent, ~E14.5), through embryonic (E15.5 to E20.5) and postnatal (P0 to P9) stages, and into their adult form. As differentiation proceeds, hair cells acquire increasing numbers of characteristic markers (represented in red for IHCs and green for OHCs; nuclei are represented as blue disks). The course of normal development is indicated with black arrows. IHCs express TBX2 from their onset and throughout life. OHCs express INSM1 and BCL11b transiently during embryonic and early postnatal stages. Transitions due to genetic manipulations are indicated by colored arrows: red if leading to an IHC-like differentiation, and green if leading to an OHC-like differentiation. (1) Removal of INSM1 from nascent OHCs (-INSM1) results in about half of them expressing TBX2 and transdifferentiating into early IHCs (red arrow), which then proceed to differentiate into mature IHCs. The other half of INSM1 lacking OHCs do not express TBX2 and proceed to differentiate into mature OHCs (green). Note that, subsequent to its deletion, there is no expression of INSM1 (whose lable has been kept in the illustration for simplicity). (2) Removal of TBX2 from the onset of IHC formation (-TBX2) results in its switching to differentiate like an OHC, transiently expressing early markers INSM1 and BCL11b, and eventually maturing into OHCs like those of adults (albeit in the position of the IHCs, and hence termed ic-OHCs). (3) Removal of TBX2 from a postnatal (up to P9) IHC (-TBX2) results in its direct transdifferentiation into a differentiated OHC, without expressing early OHC markers INSM1 and BCL11b, and transiently co-expressing markers of both mature IHCs (VGLUT3) and OHCs (PMCA2 and Prestin), before proceeding to becoming OHC-like in most respects. (4) Ectopic expression of TBX2 in late embryonic OHCs (+TBX2) results in their transdifferentiation into IHCs (expressing VGLUT3 but not PMCA2 or Prestin). (5) In the absence of both INSM1 and TBX2, nascent IHCs (-INSM1 -TBX2) transdifferentiate into OHCs whereas nascent OHCs do not transdifferentiate into IHCs. In other words, Tbx2 is epistatic to Insm1.
Supplementary information
Rights and permissions
About this article
Cite this article
García-Añoveros, J., Clancy, J.C., Foo, C.Z. et al. Tbx2 is a master regulator of inner versus outer hair cell differentiation. Nature 605, 298–303 (2022). https://doi.org/10.1038/s41586-022-04668-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04668-3
This article is cited by
-
Stem Cell-Based Hair Cell Regeneration and Therapy in the Inner Ear
Neuroscience Bulletin (2024)
-
Fgf8P2A-3×GFP/+: A New Genetic Mouse Model for Specifically Labeling and Sorting Cochlear Inner Hair Cells
Neuroscience Bulletin (2023)
-
TBX2 specifies and maintains inner hair and supporting cell fate in the Organ of Corti
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.