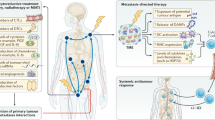
Abstract
More than 40% of men with intermediate-risk or high-risk prostate cancer will experience a biochemical recurrence after radical prostatectomy. Clinical guidelines for the management of these patients largely focus on the use of salvage radiotherapy with or without systemic therapy. However, not all patients with biochemical recurrence will go on to develop metastases or die from their disease. The optimal pre-salvage therapy investigational workup for patients who experience biochemical recurrence should, therefore, include novel techniques such as PET imaging and genomic analysis of radical prostatectomy specimen tissue, as well as consideration of more traditional clinical variables such as PSA value, PSA kinetics, Gleason score and pathological stage of disease. In patients without metastatic disease, the only known curative intervention is salvage radiotherapy but, given the therapeutic burden of this treatment, importance must be placed on accurate timing of treatment, radiation dose, fractionation and field size. Systemic therapy also has a role in the salvage setting, both concurrently with radiotherapy and as salvage monotherapy.
Key points
-
Clinical markers of local versus distant relapse of prostate cancer following radical prostatectomy include PSA level and kinetics, pathological characteristics, genomic risk scores and imaging findings.
-
During consultation after prostatectomy, clinicians should evaluate the PSA level, the PSA doubling time, the interval to biochemical failure (PSA rises to >0.2 ng/ml), and patient comorbidities, urinary bother, continence, erectile function, changes in symptoms over time, medications and genomic risk (if possible). CT and bone scan imaging is inaccurate with PSA <5 ng/ml, and so should be avoided.
-
The ideal patients for observation are: elderly (for example, age >80), low Gleason score (for example, 6–7 (International Society of Urological Pathology grade group 1–2), long PSA doubling time (for example, >12–18 months), long interval to biochemical failure (for example, >5–10 years), low absolute PSA at time of recurrence (for example, <0.5 ng/ml), multiple medical comorbidities, high risk of death from competing causes, no distant metastases on imaging.
-
Patients with high-risk features might be good candidates for salvage radiotherapy. Androgen deprivation therapy (ADT) should be considered for those patients with PSA >0.5 ng/ml.
-
Patients with documented metastases should receive ADT alone, although patients with other high-risk features might also benefit from ADT as these features could suggest occult metastatic disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Schaeffer, E. et al. NCCN guidelines insights: prostate cancer, version 1.2021. J. Natl Compr. Cancer Network 19, 134–143 (2021).
Greenberger, B. A., Zaorsky, N. G. & Den, R. B. Comparison of radical prostatectomy versus radiation and androgen deprivation therapy strategies as primary treatment for high-risk localized prostate cancer: a systematic review and meta-analysis. Eur. Urol. Focus. 6, 404–418 (2020).
Kalbasi, A. et al. Low rates of adjuvant radiation in patients with nonmetastatic prostate cancer with high-risk pathologic features. Cancer 120, 3089–3096 (2014).
Sanmamed, N. et al. Use of combined androgen deprivation therapy with postoperative radiation treatment for prostate cancer: impact of randomized trials on clinical practice. Urol. Oncol. 38, 848 e841–848.e7 (2020).
Spratt, D. E. et al. A systematic review and framework for the use of hormone therapy with salvage radiation therapy for recurrent prostate cancer. Eur. Urol. 73, 156–165 (2018).
Pisansky, T. M., Thompson, I. M., Valicenti, R. K., D’Amico, A. V. & Selvarajah, S. Adjuvant and salvage radiation therapy after prostatectomy: ASTRO/AUA guideline amendment, executive summary 2018. Pract. Radiat. Oncol. 9, 208–213 (2019).
Mottet, N. et al. EAU Guidelines — Prostate Cancer https://uroweb.org/guideline/prostate-cancer/ (2020).
Xie, W. et al. Event-free survival, a prostate-specific antigen-based composite end point, is not a surrogate for overall survival in men with localized prostate cancer treated with radiation. J. Clin. Oncol. 38, 3032–3041 (2020).
Italiano, A. Prognostic or predictive? It’s time to get back to definitions! J. Clin. Oncol. 29, 4718 (2011).
Van den Broeck, T. et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: a systematic review. Eur. Urol. 75, 967–987 (2019).
Suardi, N. et al. A nomogram predicting long-term biochemical recurrence after radical prostatectomy. Cancer 112, 1254–1263 (2008).
Amling, C. L., Bergstralh, E. J., Blute, M. L., Slezak, J. M. & Zincke, H. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J. Urol. 165, 1146–1151 (2001).
Lu-Yao, G., Stukel, T. A. & Yao, S. L. Changing patterns in competing causes of death in men with prostate cancer: a population based study. J. Urol. 171, 2285–2290 (2004).
Brockman, J. A. et al. Nomogram predicting prostate cancer-specific mortality for men with biochemical recurrence after radical prostatectomy. Eur. Urol. 67, 1160–1167 (2015).
Stoltzfus, K. C. et al. Fatal heart disease among cancer patients. Nat. Commun. 11, 2011 (2020).
Zaorsky, N. G. et al. Stroke among cancer patients. Nat. Commun. 10, 5172 (2019).
Sturgeon, K. M. et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur. Heart J. 40, 3889–3897 (2019).
Pound, C. R. et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281, 1591–1597 (1999).
Freedland, S. J. et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. J. Am. Med. Assoc. 294, 433–439 (2005).
Pompe, R. S. et al. Long-term cancer control outcomes in patients with biochemical recurrence and the impact of time from radical prostatectomy to biochemical recurrence. Prostate 78, 676–681 (2018).
Antonarakis, E. S. et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 109, 32–39 (2012).
Stamey, T. A. et al. Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. II. Radical prostatectomy treated patients. J. Urol. 141, 1076–1083 (1989).
Ploussard, G. et al. Predictive factors of oncologic outcomes in patients who do not achieve undetectable prostate specific antigen after radical prostatectomy. J. Urol. 190, 1750–1756 (2013).
Wiegel, T. et al. Prostate-specific antigen persistence after radical prostatectomy as a predictive factor of clinical relapse-free survival and overall survival: 10-year data of the ARO 96-02 trial. Int. J. Radiat. Oncol. Biol. Phys. 91, 288–294 (2015).
Wiegel, T. et al. Achieving an undetectable PSA after radiotherapy for biochemical progression after radical prostatectomy is an independent predictor of biochemical outcome–results of a retrospective study. Int. J. Radiat. Oncol. Biol. Phys. 73, 1009–1016 (2009).
Preisser, F. et al. Persistent prostate-specific antigen after radical prostatectomy and its impact on oncologic outcomes. Eur. Urol. 76, 106–114 (2019).
Fossati, N. et al. Impact of early salvage radiation therapy in patients with persistently elevated or rising prostate-specific antigen after radical prostatectomy. Eur. Urol. 7, 436–444 (2017).
Moreira, D. M. et al. Natural history of persistently elevated prostate specific antigen after radical prostatectomy: results from the SEARCH database. J. Urol. 182, 2250–2255 (2009).
Calais, J. et al. (68)Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/ml: impact on salvage radiotherapy planning. J. Nucl. Med. 59, 230–237 (2018).
Hofman, M. S. et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 395, 1208–1216 (2020).
Ma, T. M. et al. Identifying the best candidates for prostate-specific membrane antigen positron emission tomography/computed tomography as the primary staging approach among men with high-risk prostate cancer and negative conventional imaging. Eur. Urol Oncol. https://doi.org/10.1016/j.euo.2021.01.006 (2021).
Tilki, D., Preisser, F., Graefen, M., Huland, H. & Pompe, R. S. External validation of the European Association of Urology biochemical recurrence risk groups to predict metastasis and mortality after radical prostatectomy in a European cohort. Eur. Urol. 75, 896–900 (2019).
Freedland, S. J. et al. Death in patients with recurrent prostate cancer after radical prostatectomy: prostate-specific antigen doubling time subgroups and their associated contributions to all-cause mortality. J. Clin. Oncol. 25, 1765–1771 (2007).
Freedland, S. J., Humphreys, E. B., Mangold, L. A., Eisenberger, M. & Partin, A. W. Time to prostate specific antigen recurrence after radical prostatectomy and risk of prostate cancer specific mortality. J. Urol. 176, 1404–1408 (2006).
Chang, S. L., Harshman, L. C. & Presti, J. C. Jr. Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. J. Clin. Oncol. 28, 3951–3957 (2010).
Zaorsky, N. G., Buyyounouski, M. K., Li, T. & Horwitz, E. M. Aspirin and statin nonuse associated with early biochemical failure after prostate radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 84, e13–17 (2012).
Murtola, T. J. et al. Prostate cancer and PSA among statin users in the Finnish prostate cancer screening trial. Int. J. Cancer 127, 1650–1659 (2010).
Algotar, A. M., Behnejad, R., Stratton, M. S. & Stratton, S. P. Chronic use of NSAIDs and/or statins does not affect PSA or PSA velocity in men at high risk for prostate cancer. Cancer Epidemiol. Biomarkers Prevent. 23, 2196–2198 (2014).
Cher, M. L. et al. Limited role of radionuclide bone scintigraphy in patients with prostate specific antigen elevations after radical prostatectomy. J. Urol. 160, 1387–1391 (1998).
Kane, C. J. et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology 61, 607–611 (2003).
Kramer, S. et al. Sensitivity of computed tomography in detecting local recurrence of prostatic carcinoma following radical prostatectomy. Br. J. Radiol. 70, 995–999 (1997).
Calais, J., Cao, M. & Nickols, N. G. The utility of PET/CT in the planning of external radiation therapy for prostate cancer. J. Nucl. Med. 59, 557–567 (2018).
De Visschere, P. J. L. et al. A systematic review on the role of imaging in early recurrent prostate cancer. Eur. Urol. Oncol. 2, 47–76 (2019).
Jilg, C. A. et al. Diagnostic accuracy of Ga-68-HBED-CC-PSMA-Ligand-PET/CT before salvage lymph node dissection for recurrent prostate cancer. Theranostics 7, 1770–1780 (2017).
Clezardin, P. et al. Bone metastasis: mechanisms, therapies, and biomarkers. Physiol. Rev. 101, 797–855 (2021).
Bernard, S., Walker, E. & Raghavan, M. An approach to the evaluation of incidentally identified bone lesions encountered on imaging studies. Am. J. Roentgenol. 208, 960–970 (2017).
Yamaguchi, T. et al. Intertrabecular pattern of tumors metastatic to bone. Cancer 78, 1388–1394 (1996).
Clarke, N. W., McClure, J. & George, N. J. Morphometric evidence for bone resorption and replacement in prostate cancer. Br. J. Urol. 68, 74–80 (1991).
Ryan, C. et al. Epidemiology of bone metastases. Bone https://doi.org/10.1016/j.bone.2020.115783 (2020).
Zhang, X. Interactions between cancer cells and bone microenvironment promote bone metastasis in prostate cancer. Cancer Commun. 39, 76 (2019).
Horn, S. R. et al. Epidemiology of liver metastases. Cancer Epidemiol. 67, 101760 (2020).
Ciriaco, P. et al. Safety and early oncologic outcomes of lung resection in patients with isolated pulmonary recurrent prostate cancer: a single-center experience. Eur. Urol. 75, 871–874 (2019).
Polverari, G. et al. Solitary mucinous prostate adenocarcinoma lung metastasis detected by 68Ga-PSMA-11 PET/CT. Clin. Genitourin. Cancer 17, e53–e55 (2019).
Pond, G. R. et al. The prognostic importance of metastatic site in men with metastatic castration-resistant prostate cancer. Eur. Urol. 65, 3–6 (2014).
Vinjamoori, A. H. et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. Am. J. Roentgenol. 199, 367–372 (2012).
Johnstone, P. A. et al. Yield of imaging and scintigraphy assessing biochemical failure in prostate cancer patients. Urol. Oncol. 3, 108–112 (1997).
Jadvar, H. et al. Appropriate use criteria for imaging evaluation of biochemical recurrence of prostate cancer after definitive primary treatment. J. Nucl. Med. 61, 552–562 (2020).
Martino, P. et al. Role of imaging and biopsy to assess local recurrence after definitive treatment for prostate carcinoma (surgery, radiotherapy, cryotherapy, HIFU). World J. Urol. 29, 595–605 (2011).
Love, C., Din, A. S., Tomas, M. B., Kalapparambath, T. P. & Palestro, C. J. Radionuclide bone imaging: an illustrative review. Radiographics 23, 341–358 (2003).
Wong, S. K. et al. Prostate cancer and bone metastases: the underlying mechanisms. Int. J. Mol. Sci. 20, 2587 (2019).
Jin, J. K., Dayyani, F. & Gallick, G. E. Steps in prostate cancer progression that lead to bone metastasis. Int. J. Cancer 128, 2545–2561 (2011).
Adams, C. & Banks, K. P. Bone scan. In StatPearls [Internet] (StatPearls Publishing, Treasure Island, 2021).
Zhou, J. et al. Comparison of PSMA-PET/CT, choline-PET/CT, NaF-PET/CT, MRI, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a systematic review and meta-analysis. Skeletal Radiol. 48, 1915–1924 (2019).
Lengana, T. et al. (68)Ga-PSMA PET/CT replacing bone scan in the initial staging of skeletal metastasis in prostate cancer: a fait accompli? Clin. Genitourin. Cancer 16, 392–401 (2018).
Dotan, Z. A. et al. Pattern of prostate-specific antigen (PSA) failure dictates the probability of a positive bone scan in patients with an increasing PSA after radical prostatectomy. J. Clin. Oncol. 23, 1962–1968 (2005).
Pomykala, K. L. et al. Total-body (68)Ga-PSMA-11 PET/CT for bone metastasis detection in prostate cancer patients: potential impact on bone scan guidelines. J. Nucl. Med. 61, 405–411 (2020).
Heidenreich, A. et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur. Urol. 59, 61–71 (2011).
NICE Guideline Updates Team (UK) Prostate cancer: diagnosis and management. BJU Int. 124, 9–26 (2019).
Liauw, S. L. et al. Evaluation of the prostate bed for local recurrence after radical prostatectomy using endorectal magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 85, 378–384 (2013).
Robertson, N. L. et al. Combined whole body and multiparametric prostate magnetic resonance imaging as a 1-step approach to the simultaneous assessment of local recurrence and metastatic disease after radical prostatectomy. J. Urol. 198, 65–70 (2017).
Thoeny, H. C. et al. Metastases in normal-sized pelvic lymph nodes: detection with diffusion-weighted MR imaging. Radiology 273, 125–135 (2014).
Caglic, I. & Barrett, T. Diffusion-weighted imaging (DWI) in lymph node staging for prostate cancer. Transl. Androl. Urol. 7, 814–823 (2018).
Lecouvet, F. E. et al. Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: diagnostic and cost-effectiveness and comparison with current detection strategies. J. Clin. Oncol. 25, 3281–3287 (2007).
Woo, S., Suh, C. H., Kim, S. Y., Cho, J. Y. & Kim, S. H. Diagnostic performance of magnetic resonance imaging for the detection of bone metastasis in prostate cancer: a systematic review and meta-analysis. Eur. Urol. 73, 81–91 (2018).
Sharma, V. et al. Multiparametric magnetic resonance imaging is an independent predictor of salvage radiotherapy outcomes after radical prostatectomy. Eur. Urol. 73, 879–887 (2018).
Czernin, J., Satyamurthy, N. & Schiepers, C. Molecular mechanisms of bone 18F-NaF deposition. J. Nucl. Med. 51, 1826–1829 (2010).
Fonager, R. F. et al. Prospective comparative study of (18)F-sodium fluoride PET/CT and planar bone scintigraphy for treatment response assessment of bone metastases in patients with prostate cancer. Acta Oncol. 57, 1063–1069 (2018).
Jambor, I. et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 55, 59–67 (2016).
Langsteger, W., Rezaee, A., Pirich, C. & Beheshti, M. 18F-NaF-PET/CT and 99mTc-MDP bone scintigraphy in the detection of bone metastases in prostate cancer. Semin. Nucl. Med. 46, 491–501 (2016).
Wondergem, M. et al. 99mTc-HDP bone scintigraphy and 18F-sodiumfluoride PET/CT in primary staging of patients with prostate cancer. World J. Urol. 36, 27–34 (2018).
Zacho, H. D. et al. Prospective comparison of 68Ga-PSMA PET/CT, 18F-sodium fluoride PET/CT and diffusion weighted-MRI at for the detection of bone metastases in biochemically recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 45, 1884–1897 (2018).
Dyrberg, E. et al. 68Ga-PSMA-PET/CT in comparison wh 18F-fluoride-PET/CT and whole-body MRI for the detection of bone metastases in patients with prostate cancer: a prospective diagnostic accuracy study. Eur. Radiol. 29, 1221–1230 (2019).
Hillner, B. E. et al. 18F-fluoride PET used for treatment monitoring of systemic cancer therapy: results from the national oncologic PET registry. J. Nucl. Med. 56, 222–228 (2015).
Hillner, B. E. et al. Impact of 18F-fluoride PET in patients with known prostate cancer: initial results from the national oncologic PET registry. J. Nucl. Med. 55, 574–581 (2014).
Jensen, T. S. Decision Memo for Positron Emission Tomography (NaF-18) to Identify Bone Metastasis of Cancer (CAG-00065R2), https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=279 (2021).
Forrest, W. CMS again declines coverage for NaF-PET scans. https://www.auntminnie.com/index.aspx?sec=ser&sub=def&pag=dis&ItemID=120979 (2018).
Perera, M. et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur. Urol. 77, 403–417 (2020).
Eiber, M. et al. Prostate-specific membrane antigen ligands for imaging and therapy. J. Nucl. Med. 58, 67S–76S (2017).
Eder, M. et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 23, 688–697 (2012).
FDA. Full prescribing information: Choline 11 C. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203155s000lbl.pdf (2021).
Mapelli, P. et al. 11C- or 18F-choline PET/CT for imaging evaluation of biochemical recurrence of prostate cancer. J. Nucl. Med. 57, 43S–48S (2016).
Duncan, K. Radiopharmaceuticals in PET imaging. J. Nucl. Med. Technol. 26, 228–234; quiz 242 (1998).
Vargas, H. A. et al. Localizing sites of disease in patients with rising serum prostate-specific antigen up to 1ng/ml following prostatectomy: how much information can conventional imaging provide? Urol. Oncol. 34, 482 e485–482.e10 (2016).
Yoon, J., Ballas, L., Desai, B. & Jadvar, H. Prostate-specific antigen and prostate-specific antigen kinetics in predicting 18F-sodium fluoride positron emission tomography-computed tomography positivity for first bone metastases in patients with biochemical recurrence after radical prostatectomy. World J. Nucl. Med. 16, 229–236 (2017).
Lavallee, E. et al. Increased prostate cancer glucose metabolism detected by 18F-fluorodeoxyglucose positron emission tomography/computed tomography in localised gleason 8–10 prostate cancers identifies very high-risk patients for early recurrence and resistance to castration. Eur. Urol. Focus 5, 998–1006 (2019).
Wibmer, A. G. et al. Quantification of metastatic prostate cancer whole-body tumor burden with fdg pet parameters and associations with overall survival after first line abiraterone or enzalutamide: a single-center retrospective cohort study. J. Nucl. Med. https://doi.org/10.2967/jnumed.120.256602 (2021).
Wang, B. et al. A prospective trial of 68Ga-PSMA and 18F-FDG PET/CT in nonmetastatic prostate cancer patients with an early PSA progression during castration. Clin. Cancer Res. 26, 4551–4558 (2020).
Fox, J. J. et al. Positron emission tomography/computed tomography-based assessments of androgen receptor expression and glycolytic activity as a prognostic biomarker for metastatic castration-resistant prostate cancer. JAMA Oncol. 4, 217–224 (2018).
Calais, J. et al. Resection of a solitary pulmonary metastasis from prostatic Adenocarcinoma misdiagnosed as a bronchocele: usefulness of 18F-choline and 18F-FDG PET/CT. J. Thorac. Oncol. 9, 1826–1829 (2014).
Fanti, S. et al. PET/CT with 11C-choline for evaluation of prostate cancer patients with biochemical recurrence: meta-analysis and critical review of available data. Eur. J. Nucl. Med. Mol. Imaging 43, 55–69 (2016).
Nanni, C. et al. 18F-FACBC (anti1-amino-3-18F-fluorocyclobutane-1-carboxylic acid) versus 11C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur. J. Nucl. Med. Mol. Imaging 43, 1601–1610 (2016).
Fossati, N. et al. Underestimation of PET/CT scan in assessing tumour burden of men with nodal recurrence from prostate cancer: head-to-head comparison of 68Ga-PSMA and 11C-choline in a large, multi-institutional series of extended salvage lymph node dissections. J. Urol. 204, 296–302 (2020).
Emmett, L. et al. Prospective, multisite, international comparison of 18F-fluoromethylcholine PET/CT, multiparametric MRI, and 68Ga-HBED-CC PSMA-11 PET/CT in men with high-risk features and biochemical failure after radical prostatectomy: clinical performance and patient outcomes. J. Nucl. Med. 60, 794–800 (2019).
Cantiello, F. et al. Comparison between 64Cu-PSMA-617 PET/CT and 18F-choline PET/CT imaging in early diagnosis of prostate cancer biochemical recurrence. Clin. Genitourin. Cancer 16, 385–391 (2018).
Soydal, C. et al. Comparison of bone scintigraphy and Ga-68 prostate-specific membrane antigen positron emission tomography/computed tomography in the detection of bone metastases of prostate carcinoma. Nucl. Med. Commun. 40, 1243–1249 (2019).
Witkowska-Patena, E. et al. Head-to-head comparison of 18F-prostate-specific membrane antigen-1007 and 18F-fluorocholine PET/CT in biochemically relapsed prostate cancer. Clin. Nucl. Med. 44, e629–e633 (2019).
Morigi, J. J. et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J. Nucl. Med. 56, 1185–1190 (2015).
Grassi, I. et al. The clinical use of PET with 11C-acetate. Am. J. Nucl. Med. Mol. Imaging 2, 33–47 (2012).
Regula, N. et al. Comparison of 68Ga-PSMA-11 PET/CT with 11C-acetate PET/CT in re-staging of prostate cancer relapse. Sci. Rep. 10, 4993 (2020).
Schuster, D. M., Nanni, C. & Fanti, S. Evaluation of prostate cancer with radiolabeled amino acid analogs. J. Nucl. Med. 57, 61S–66S (2016).
Savir-Baruch, B. et al. ACR-ACNM practice parameter for the performance of fluorine-18 fluciclovine-PET/CT for recurrent prostate cancer. Clin. Nucl. Med. 43, 909–917 (2018).
Calais, J. et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 20, 1286–1294 (2019).
England, J. R., Paluch, J., Ballas, L. K. & Jadvar, H. 18F-Fluciclovine PET/CT detection of recurrent prostate carcinoma in patients with serum PSA </= 1 ng/mL after definitive primary treatment. Clin. Nucl. Med. 44, e128–e132 (2019).
Jadvar, H. et al. Baseline 18F-FDG PET/CT parameters as imaging biomarkers of overall survival in castrate-resistant metastatic prostate cancer. J. Nucl. Med. 54, 1195–1201 (2013).
Schuster, D. M. et al. Detection of recurrent prostate carcinoma with anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT and 111In–capromab pendetide SPECT/CT. Radiology 259, 852–861 (2011).
Calais, J. et al. What is the best PET target for early biochemical recurrence of prostate cancer? — Authors’ reply. Lancet Oncol. 20, e609–e610 (2019).
Jilg, C. A. et al. Detection rate of 18F-choline PET/CT and 68Ga-PSMA-HBED-CC PET/CT for prostate cancer lymph node metastases with direct link from PET to histopathology: dependence on the size of tumor deposits in lymph nodes. J. Nucl. Med. 60, 971–977 (2019).
Bronsert, P., Reichel, K. & Ruf, J. Loss of PSMA expression in non-neuroendocrine dedifferentiated acinar prostate cancer. Clin. Nucl. Med. 43, 526–528 (2018).
Bakht, M. K. et al. Neuroendocrine differentiation of prostate cancer leads to PSMA suppression. Endocr. Relat. Cancer 26, 131–146 (2018).
Chu, C. E. et al. Prostate-specific membrane antigen and fluciclovine transporter genes are associated with variable clinical features and molecular subtypes of primary prostate cancer. Eur. Urol. 79, 717–721 (2021).
Rischpler, C. et al. 68Ga-PSMA-HBED-CC uptake in cervical, celiac, and sacral ganglia as an important pitfall in prostate cancer PET imaging. J. Nucl. Med. 59, 1406–1411 (2018).
Sheikhbahaei, S. et al. Prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer: an update on important pitfalls. Semin. Nucl. Med. 49, 255–270 (2019).
Rousseau, E. et al. A prospective study on 18F-DCFPyL PSMA PET/CT imaging in biochemical recurrence of prostate cancer. J. Nucl. Med. 60, 1587–1593 (2019).
Treglia, G. et al. Detection rate of 18F-labeled PSMA PET/CT in biochemical recurrent prostate cancer: a systematic review and a meta-analysis. Cancers 11, 710 (2019).
Wondergem, M. et al. Early lesion detection with 18F-DCFPyL PET/CT in 248 patients with biochemically recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 46, 1911–1918 (2019).
Giesel, F. L. et al. Detection efficacy of 18F-PSMA-1007 PET/CT in 251 patients with biochemical recurrence of prostate cancer after radical prostatectomy. J. Nucl. Med. 60, 362–368 (2019).
Rowe, S. P. et al. Prospective evaluation of PSMA-targeted 18F-DCFPyL PET/CT in men with biochemical failure after radical prostatectomy for prostate cancer. J. Nucl. Med. 61, 58–61 (2020).
Giesel, F. L. et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 44, 678–688 (2017).
FDA. FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer (2020).
Lantheus. Lantheus Receives U.S. FDA Approval of PYLARIFY® (piflufolastat F 18) Injection, the First and Only Commercially Available PSMA PET Imaging Agent for Prostate Cancer. https://investor.lantheus.com/news-releases/news-release-details/lantheus-receives-us-fda-approval-pylarifyr-piflufolastat-f-18 (2021).
Wiltshire, K. L. et al. Anatomic boundaries of the clinical target volume (prostate bed) after radical prostatectomy. Int. J. Radiat. Oncol. Biol. Phys. 69, 1090–1099 (2007).
van Kalmthout, L. W. M. et al. Prospective validation of gallium-68 prostate specific membrane antigen-positron emission tomography/computerized tomography for primary staging of prostate cancer. J. Urol. 203, 537–545 (2020).
Calais, J., Czernin, J., Fendler, W. P., Elashoff, D. & Nickols, N. G. Randomized prospective phase III trial of 68Ga-PSMA-11 PET/CT molecular imaging for prostate cancer salvage radiotherapy planning [PSMA-SRT]. BMC Cancer 19, 18 (2019).
Zschaeck, S. et al. Intermediate-term outcome after PSMA-PET guided high-dose radiotherapy of recurrent high-risk prostate cancer patients. Radiat. Oncol. 12, 140 (2017).
Schmidt-Hegemann, N. S. et al. Outcome after PSMA PET/CT based radiotherapy in patients with biochemical persistence or recurrence after radical prostatectomy. Radiat. Oncol. 13, 37 (2018).
Fendler, W. P. et al. Assessment of 68Ga-PSMA-11 pet accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 5, 856–863 (2019).
Emmett, L. et al. 3-year freedom from progression following 68GaPSMA PET CT triaged management in men with biochemical recurrence post radical prostatectomy. Results of a prospective multi-center trial. J. Nucl. Med. 203, 1063 (2019).
Hricak, H. et al. Medical imaging and nuclear medicine: a Lancet Oncology commission. Lancet Oncol. 22, e136–e172 (2021).
Lehrer, E. J. et al. Safety and survival rates associated with ablative stereotactic radiotherapy for patients with oligometastatic cancer: a systematic review and meta-analysis. JAMA Oncol. 7, 92–106 (2021).
Zaorsky, N. G. et al. Impact of radiation therapy dose escalation on prostate cancer outcomes and toxicities. Am. J. Clin. Oncol. 41, 409–415 (2018).
Lehrer, E. J. et al. Ultrahypofractionated versus hypofractionated and conventionally fractionated radiation therapy for localized prostate cancer: a systematic review and meta-analysis of phase III randomized trials. Radiother. Oncol. 148, 235–242 (2020).
Levin-Epstein, R. G. et al. Dose-response with stereotactic body radiotherapy for prostate cancer: a multi-institutional analysis of prostate-specific antigen kinetics and biochemical control. Radiother. Oncol. 154, 207–213 (2021).
Parker, C. C. et al. Timing of radiotherapy after radical prostatectomy (RADICALS-RT): a randomised, controlled phase 3 trial. Lancet 396, 1413–1421 (2020).
Sargos, P. et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): a randomised, phase 3 trial. Lancet Oncol. 21, 1341–1352 (2020).
Kneebone, A. et al. Adjuvant radiotherapy versus early salvage radiotherapy following radical prostatectomy (TROG 08.03/ANZUP RAVES): a randomised, controlled, phase 3, non-inferiority trial. Lancet Oncol. 21, 1331–1340 (2020).
Vale, C. L. et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet 396, 1422–1431 (2020).
Trock, B. J. et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 299, 2760–2769 (2008).
Nehra, A. et al. Identification of recurrence sites following post-prostatectomy treatment for prostate cancer using 11C-choline positron emission tomography and multiparametric pelvic magnetic resonance imaging. J. Urol. 199, 726–733 (2018).
Hawken, S. R. et al. Utilization of salvage radiation therapy for biochemical recurrence after radical prostatectomy. Int. J. Radiat. Oncol. Biol. Phys. 104, 1030–1034 (2019).
Morgan, T. M. et al. Variation in the use of postoperative radiotherapy among high-risk patients following radical prostatectomy. Prostate Cancer Prostatic Dis. 19, 216–221 (2016).
Yokomizo, A. et al. Salvage radiotherapy versus hormone therapy for prostate-specific antigen failure after radical prostatectomy: a randomised, multicentre, open-label, phase 3 trial (JCOG0401)†. Eur. Urol. 77, 689–698 (2019).
Duchesne, G. M. et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 17, 727–737 (2016).
Carrie, C. et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 17, 747–756 (2016).
Carrie, C. et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 20, 1740–1749 (2019).
Shipley, W. U. et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N. Engl. J. Med. 376, 417–428 (2017).
Pollack, A. et al. Short term androgen deprivation therapy without or with pelvic lymph node treatment added to prostate bed only salvage radiation therapy: the NRG Oncology/RTOG 0534 SPPORT trial. Int. J. Radiat. Oncol. Biol. Phys. 2, 1393–1610 (2018).
Michalski, J. M. et al. Effect of standard vs dose-escalated radiation therapy for patients with intermediate-risk prostate cancer: the NRG oncology RTOG 0126 randomized clinical trial. JAMA Oncol. 4, e180039 (2018).
Rodda, S., Morris, W. J., Hamm, J. & Duncan, G. ASCENDE-RT: an analysis of health-related quality of life for a randomized trial comparing low-dose-rate brachytherapy boost with dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 98, 581–589 (2017).
Zaorsky, N. G. et al. What is the ideal radiotherapy dose to treat prostate cancer? A meta-analysis of biologically equivalent dose escalation. Radiother. Oncol. 115, 295–300 (2015).
Dearnaley, D. et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 17, 1047–1060 (2016).
Widmark, A. et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 394, 385–395 (2019).
Catton, C. N. et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J. Clin. Oncol. 35, 1884–1890 (2017).
Roach, M. et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): long-term results of a randomised, phase 3 trial. Lancet Oncol. 19, 1504–1515 (2018).
Johnson, M. E. et al. Patient reported outcomes among treatment modalities for prostate cancer. Can. J. Urol. 23, 8535–8545 (2016).
Donovan, J. L. et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N. Engl. J. Med. 375, 1425–1437 (2016).
Bolla, M. et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 360, 103–106 (2002).
Zaorsky, N. G., Spratt, D. E., Kishan, A. U., Culp, S. H. & Showalter, T. N. Editorial: optimizing local therapy for high-risk prostate cancer: evidence and emerging options. Front. Oncol. 10, 1616 (2020).
Zaorsky, N. G., Trabulsi, E. J., Lin, J. & Den, R. B. Multimodality therapy for patients with high-risk prostate cancer: current status and future directions. Semin. Oncol. 40, 308–321 (2013).
Tendulkar, R. D. et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J. Clin. Oncol. 34, 3648–3654 (2016).
Stephenson, A. J. et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J. Clin. Oncol. 25, 2035–2041 (2007).
Kishan, A. U. et al. Optimizing the timing of salvage postprostatectomy radiotherapy and the use of concurrent hormonal therapy for prostate cancer. Eur. Urol. Oncol. 1, 3–18 (2018).
Stish, B. J. et al. Improved Metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J. Clin. Oncol. 34, 3864–3871 (2016).
Dess, R. T. et al. Association of presalvage radiotherapy PSA levels after prostatectomy with outcomes of long-term antiandrogen therapy in men with prostate cancer. JAMA Oncol. 6, 735–743 (2020).
Cornford, P. et al. EAU-ESTRO-SIOG guidelines on prostate cancer. part ii: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 71, 630–642 (2017).
Valicenti, R. K. et al. Adjuvant and salvage radiation therapy after prostatectomy: American Society for Radiation Oncology/American Urological Association guidelines. Int. J. Radiat. Oncol. Biol. Phys. 86, 822–828 (2013).
King, C. R. The timing of salvage radiotherapy after radical prostatectomy: a systematic review. Int. J. Radiat. Oncol. Biol. Phys. 84, 104–111 (2012).
Boorjian, S. A. et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur. Urol. 59, 893–899 (2011).
Jackson, W. C. et al. A prostate-specific antigen doubling time of <6 months is prognostic for metastasis and prostate cancer-specific death for patients receiving salvage radiation therapy post radical prostatectomy. Radiat. Oncol. 8, 170 (2013).
van Stam, M. A. et al. The effect of salvage radiotherapy and its timing on the health-related quality of life of prostate cancer patients. Eur. Urol. 70, 751–757 (2016).
Cozzarini, C. et al. Clinical factors predicting late severe urinary toxicity after postoperative radiotherapy for prostate carcinoma: a single-institute analysis of 742 patients. Int. J. Radiat. Oncol. Biol. Phys. 82, 191–199 (2016).
Nyarangi-Dix, J. N. et al. Post-prostatectomy radiotherapy adversely affects urinary continence irrespective of radiotherapy regime. World J. Urol. 35, 1841–1847 (2016).
De Meerleer, G. et al. Salvage intensity-modulated radiotherapy for rising PSA after radical prostatectomy. Radiother. Oncol. 89, 205–213 (2008).
Goenka, A. et al. Improved toxicity profile following high-dose postprostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur. Urol. 60, 1142–1148 (2011).
Berlin, A. et al. Phase 2 trial of guideline-based postoperative image guided intensity modulated radiation therapy for prostate cancer: toxicity, biochemical, and patient-reported health-related quality-of-life outcomes. Practical Radiat. Oncol. 5, e473–482 (2015).
Tendulkar, R. D. et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J. Clin. Oncol. 34, 3648–3654 (2015).
Stish, B. J. et al. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J. Clin. Oncol. 34, 3864–3871 (2015).
Fossati, N. et al. Impact of early salvage radiation therapy in patients with persistently elevated or rising prostate-specific antigen after radical prostatectomy. Eur. Urol. 73, 436–444 (2018).
Fossati, N. et al. Assessing the optimal timing for early salvage radiation therapy in patients with prostate-specific antigen rise after radical prostatectomy. Eur. Urol. 69, 728–733 (2016).
Abugharib, A. et al. Very early salvage radiotherapy improves distant metastasis-free survival. J. Urol. 197, 662–668 (2017).
Abugharib, A. et al. Very early salvage radiotherapy improves distant metastasis-free survival. J. Urol. 197, 662–668 (2017).
Seisen, T., Trinh, Q. D. & Abdollah, F. Could lead-time bias explain the apparent benefits of early salvage radiotherapy? Nat. Rev. Urol. 14, 193–194 (2017).
Pasalic, D. et al. Dose escalation for prostate adenocarcinoma: a long-term update on the outcomes of a phase 3, single institution randomized clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 104, 790–797 (2019).
King, C. R. & Kapp, D. S. Radiotherapy after prostatectomy: is the evidence for dose escalation out there? Int. J. Radiat. Oncol. Biol. Phys. 71, 346–350 (2008).
Alexidis, P. et al. Use of high and very high dose radiotherapy after radical prostatectomy for prostate cancer in the United States. Prostate Cancer Prostatic Dis. 21, 584–593 (2018).
Gharzai, L. A. et al. Intermediate clinical endpoints for surrogacy in localised prostate cancer: an aggregate meta-analysis. Lancet Oncol. 22, 402–410 (2021).
King, C. R. The dose-response of salvage radiotherapy following radical prostatectomy: A systematic review and meta-analysis. Radiother. Oncol. 121, 199–203 (2016).
Pisansky, T. M. et al. Salvage radiation therapy dose response for biochemical failure of prostate cancer after prostatectomy-a multi-institutional observational study. Int. J. Radiat. Oncol. Biol. Phys. 96, 1046–1053 (2016).
Qi, X. et al. Toxicity and biochemical outcomes of dose-intensified postoperative radiation therapy for prostate cancer: results of a randomized phase III trial. Int. J. Radiat. Oncol. Biol. Phys. 106, 282–290 (2020).
Ghadjar, P. et al. Acute toxicity and quality of life after dose-intensified salvage radiation therapy for biochemically recurrent prostate cancer after prostatectomy: first results of the randomized trial SAKK 09/10. J. Clin. Oncol. 33, 4158–4166 (2015).
Ghadjar, P. et al. Impact of dose intensified salvage radiation therapy on urinary continence recovery after radical prostatectomy: results of the randomized trial SAKK 09/10. Radiother. Oncol. 126, 257–262 (2018).
van Andel, G. et al. An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur. J. Cancer 44, 2418–2424 (2008).
Zaorsky, N. G. et al. Evolution of advanced technologies in prostate cancer radiotherapy. Nat. Rev. Urol. 10, 565–579 (2013).
Ghadjar, P. et al. Impact of dose intensified salvage radiation therapy on urinary continence recovery after radical prostatectomy: results of the randomized trial SAKK 09/10. Radiother.Oncol. 126, 257–262 (2018).
Sandler, K. A. et al. Prostate-only versus whole-pelvis radiation with or without a brachytherapy boost for gleason grade group 5 prostate cancer: a retrospective analysis. Eur. Urol. 77, 3–10 (2020).
Pommier, P. et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Update of the long-term survival results of the GETUG-01 randomized study. Int. J. Radiat. Oncol. Biol. Phys. 96, 759–769 (2016).
Nguyen, P. L. & D’Amico, A. V. Targeting pelvic lymph nodes in men with intermediate- and high-risk prostate cancer despite two negative randomized trials. J. Clin. Oncol. 26, 2055–2056 (2008).
Moghanaki, D., Urdaneta, A. I., Karlin, J. D., Koontz, B. F. & Anscher, M. S. Management of postprostatectomy biochemical relapse with salvage radiotherapy: results of an international survey. Am. J. Clin. Oncol. 39, 64–68 (2016).
Ramey, S. J. et al. Multi-institutional evaluation of elective nodal irradiation and/or androgen deprivation therapy with postprostatectomy salvage radiotherapy for prostate cancer. Eur. Urol. 74, 99–106 (2018).
Michalski, J. M. et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 76, 361–368 (2010).
Zaorsky, N. G. et al. Prostate cancer patients with unmanaged diabetes or receiving insulin experience inferior outcomes and toxicities after treatment with radiation therapy. Clin. Genitourin. Cancer 15, 326–335 e323 (2017).
Wang, L. S. et al. Impact of obesity on outcomes after definitive dose-escalated intensity-modulated radiotherapy for localized prostate cancer. Cancer 121, 3010–3017 (2015).
Hall, W. A. et al. NRG oncology updated international consensus atlas on pelvic lymph node volumes for intact and postoperative prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. (2020).
Brenner, D. J. & Hall, E. J. Fractionation and protraction for radiotherapy of prostate carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 43, 1095–1101(1999).
Zaorsky, N. G. et al. Comparison of outcomes and toxicities among radiation therapy treatment options for prostate cancer. Cancer Treat. Rev. 48, 50–60 (2016).
Morgan, S. C. et al. Hypofractionated radiation therapy for localized prostate cancer: an ASTRO, ASCO, and AUA evidence-based guideline. J. Clin. Oncol. 36, Jco1801097 (2018).
N. C. C. Network NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer, http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (2019).
Martell, K. et al. 5-Year outcomes of a prospective phase 1/2 study of accelerated hypofractionated radiation therapy to the prostate bed. Practical Radiat. Oncol. 9, 354–361 (2019).
Chin, S. et al. Ten-year outcomes of moderately hypofractionated salvage postprostatectomy radiation therapy and external validation of a contemporary multivariable nomogram for biochemical failure. Int. J. Radiat. Oncol. Biol. Phys. 107, 288–296 (2020).
Cozzarini, C. et al. Higher-than-expected severe (Grade 3–4) late urinary toxicity after postprostatectomy hypofractionated radiotherapy: a single-institution analysis of 1176 patients. Eur. Urol. 66, 1024–1030 (2014).
Wei, J. T., Dunn, R. L., Litwin, M. S., Sandler, H. M. & Sanda, M. G. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 56, 899–905 (2000).
Koerber, S. A. et al. Prostate bed irradiation with alternative radio-oncological approaches (PAROS) — a prospective, multicenter and randomized phase III trial. Radiat. Oncol. 14, 122 (2019).
Kishan, A. U. et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw. Open 2, e188006 (2019).
Brand, D. H. et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 20, 1531–1543 (2019).
Ballas, L. K. et al. Phase 1 trial of SBRT to the prostate fossa after prostatectomy. Int. J. Radiat. Oncol. Biol. Phys. 104, 50–60 (2019).
Sampath, S. et al. Stereotactic body radiation therapy to the prostate bed: results of a phase 1 dose-escalation trial. Int. J. Radiat. Oncol. Biol. Phys. 106, 537–545 (2020).
Yoon, S. et al. Prostate bed and organ-at-risk deformation: prospective volumetric and dosimetric data from a phase II trial of stereotactic body radiotherapy after radical prostatectomy. Radiother. Oncol. 148, 44–50 (2020).
Zaorsky, N. G. et al. ACR appropriateness criteria for external beam radiation therapy treatment planning for clinically localized prostate cancer, part II of II. Adv. Radiat. Oncol. 2, 437–454 (2017).
Zaorsky, N. G. et al. ACR appropriateness criteria® external beam radiation therapy treatment planning for clinically localized prostate cancer, part I of II. Adv. Radiat. Oncol. 2, 62–84 (2017).
Yang, D. D. & Nguyen, P. L. Optimizing androgen deprivation therapy with radiation therapy for aggressive localized and locally advanced prostate cancer. Urol. Oncol. https://doi.org/10.1016/j.urolonc.2017.10.020 (2017).
Polkinghorn, W. R. et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 3, 1245–1253 (2013).
Goodwin, J. F. et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 3, 1254–1271 (2013).
Burnette, B. & Weichselbaum, R. R. Radiation as an immune modulator. Semin. Radiat. Oncol. 23, 273–280 (2013).
Andre, F. et al. Molecular pathways: involvement of immune pathways in the therapeutic response and outcome in breast cancer. Clin. Cancer Res. 19, 28–33 (2013).
Kaur, P. & Asea, A. Radiation-induced effects and the immune system in cancer. Front. Oncol. 2, 191 (2012).
Wang, H. H. et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett. 375, 349–359 (2016).
Spratt, D. E. et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res. 75, 4688–4696 (2015).
Nguyen, P. L. et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur. Urol. 67, 825–836 (2015).
Dinh, K. T. et al. Association between androgen deprivation therapy and anxiety among 78 000 patients with localized prostate cancer. Int. J. Urol. 24, 743–748 (2017).
Nead, K. T. et al. Androgen deprivation therapy and future Alzheimer’s disease risk. J. Clin. Oncol. 34, 566–571 (2016).
Lapi, F. et al. Androgen deprivation therapy and risk of acute kidney injury in patients with prostate cancer. JAMA 310, 289–296 (2013).
Carrie, C. et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol. 20, 1740–1749 (2013).
Dess, R. T. et al. Association of presalvage radiotherapy PSA levels after prostatectomy with outcomes of long-term antiandrogen therapy in men with prostate cancer. JAMA Oncol. 6, 735–743 (2013).
Chen, A. B. et al. Expectations about the effectiveness of radiation therapy among patients with incurable lung cancer. J. Clin. Oncol. 31, 2730–2735 (2013).
Zaorsky, N. G. et al. What are medical students in the united states learning about radiation oncology? Results of a multi-institutional survey. Int. J. Radiat. Oncol. Biol. Phys. 94, 235–242 (2016).
Mahal, B. A. et al. Travel distance and stereotactic body radiotherapy for localized prostate cancer. Cancer 124, 1141–1149 (2018).
Tsai, H. K., D’Amico, A. V., Sadetsky, N., Chen, M. H. & Carroll, P. R. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J. Natl Cancer Inst. 99, 1516–1524 (2007).
Crook, J. M. et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N. Engl. J. Med. 367, 895–903 (2012).
Nabid, A. et al. Duration of androgen deprivation therapy in high-risk prostate cancer: a randomized phase III trial. Eur. Urol. 74, 432–441 (2018).
Spratt, D. E. et al. Development and validation of a novel integrated clinical-genomic risk group classification for localized prostate cancer. J. Clin. Oncol. 36, 581–590 (2018).
Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015).
Armenia, J. et al. The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 50, 645–651 (2018).
Spratt, D. E. Prostate cancer transcriptomic subtypes. Adv. Exp. Med. Biol. 1210, 111–120 (2019).
Jairath, N. K. et al. A systematic review of the evidence for the decipher genomic classifier in prostate cancer. Eur. Urol. 79, 374–383 (2021).
Spratt, D. E. et al. Individual patient-level meta-analysis of the performance of the decipher genomic classifier in high-risk men after prostatectomy to predict development of metastatic disease. J. Clin. Oncol. 35, 1991–1998 (2017).
Spratt, D. E. et al. Performance of a prostate cancer genomic classifier in predicting metastasis in men with prostate-specific antigen persistence postprostatectomy. Eur. Urol. 74, 107–114 (2018).
Howard, L. E. et al. Validation of a genomic classifier for prediction of metastasis and prostate cancer-specific mortality in African-American men following radical prostatectomy in an equal access healthcare setting. Prostate Cancer Prostatic Dis. 23, 419–428 (2019).
Gore, J. L. et al. Clinical utility of a genomic classifier in men undergoing radical prostatectomy: the PRO-IMPACT trial. Pract. Radiat. Oncol. 10, e82–e90 (2020).
Marascio, J. et al. Prospective study to define the clinical utility and benefit of Decipher testing in men following prostatectomy. Prostate Cancer Prostatic Dis. 23, 295–302 (2019).
FY, F. et al. Transcriptome profiling of NRG Oncology/RTOG 9601: validation of a prognostic genomic classifier in salvage radiotherapy prostate cancer patients from a prospective randomized trial. J. Clin. Oncol. https://doi.org/10.1200/JCO.2020.38.6_suppl.276 (2020).
Zhao, S. G. et al. Development and validation of a 24-gene predictor of response to postoperative radiotherapy in prostate cancer: a matched, retrospective analysis. Lancet Oncol. 17, 1612–1620 (2016).
Karnes, R. J. et al. Development and validation of a prostate cancer genomic signature that predicts early ADT treatment response following radical prostatectomy. Clin. Cancer Res. 24, 3908–3916 (2018).
Zhao, S. G. et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 3, 1663–1672 (2017).
Spratt, D. E. et al. Transcriptomic heterogeneity of androgen receptor activity defines a de novo low AR-active subclass in treatment naive primary prostate cancer. Clin. Cancer Res. 25, 6721–6730 (2019).
Zaorsky, N. G. et al. Clinical trial accrual at initial course of therapy for cancer and its impact on survival. J. Natl Compr. Cancer Netw. 17, 1309–1316 (2019).
Virgolini, I., Decristoforo, C., Haug, A., Fanti, S. & Uprimny, C. Current status of theranostics in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 45, 471–495 (2018).
Chen, Z. et al. PSMA-targeted theranostic nanoplex for prostate cancer therapy. ACS Nano 6, 7752–7762 (2012).
Fanti, S. et al. Consensus on molecular imaging and theranostics in prostate cancer. Lancet Oncol. 19, e696–e708 (2018).
Singh, R. et al. Single fraction radiosurgery, fractionated radiosurgery, and conventional radiotherapy for spinal oligometastasis (SAFFRON): a systematic review and meta-analysis. Radiother. Oncol. 146, 76–89 (2020).
Beresford, M. J., Gillatt, D., Benson, R. J. & Ajithkumar, T. A systematic review of the role of imaging before salvage radiotherapy for post-prostatectomy biochemical recurrence. Clin. Oncol. 22, 46–55 (2010).
Acknowledgements
N.G.Z. is supported by startup funding from Penn State Cancer Institute and Penn State College of Medicine, is supported by the National Institutes of Health Grant L radical prostatectomy 1 L30 CA231572-01, is supported by the American Cancer Society – Tri State CEOs Against Cancer Clinician Scientist Development Grant, CSDG-20-013-01-CCE and received remuneration from Springer Nature for his textbook, Absolute Clinical Radiation Oncology Review.
Author information
Authors and Affiliations
Contributions
N.G.Z., J.C., S.F., D.T., T.D., D.E.S. and A.U.K. researched data for the article, made a substantial contribution to the discussion of its content, wrote and reviewed the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
J.C. is supported by the Prostate Cancer Foundation (2020 Young Investigator Award 20YOUN05, 2019 Challenge Award 19CHAL02), the Society of Nuclear Medicine and Molecular imaging (2019 Molecular Imaging Research Grant for Junior Academic Faculty) and reports prior consulting activities outside of the submitted work for Advanced Accelerator Applications, Blue Earth Diagnostics, Curium Pharma, GE Healthcare, Janssen Pharmaceuticals, Progenics Pharmaceuticals, Radiomedix and Telix Pharmaceuticals. The remaining authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thank L. Emmett, M. Emberton and the other, anonymous, reviewer for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Androgen deprivation therapy
-
(ADT). Also known as hormone therapy, typically includes drugs such as LHRH agonists (e.g. leuprolide) and LHRH antagonists (e.g. degarelix). Androgen receptor inhibitors (e.g. enzalutamide) and anti-androgens (e.g. bicalutamide) technically do not decrease androgen production, and technically are not ADT, but they are commonly put in the same category. Short-term ADT implies use of ADT for 3–6 months, and long-term ADT implies use for 12–36 months.
- Biochemical recurrence
-
After prostatectomy, biochemical recurrence is historically defined by two consecutive PSA values >0.2 ng/ml. After definitive radiation therapy (i.e. for men with an intact prostate), this is defined by the nadir +2 ng/ml PSA.
- Salvage radiation therapy
-
Radiation therapy prescribed to the prostate bed, with or without the pelvic lymph nodes, in the setting of a rising PSA after prostatectomy. The historical definition for salvage radiotherapy is with PSA of ≥0.2 ng/ml with a second confirmatory value. ‘Early salvage’ refers to radiotherapy with PSA < 0.5 ng/ml. ‘Very early salvage’ refers to radiotherapy with PSA 0.01–0.2 ng/ml.
- Prostate bed
-
The prostate bed has the following boundaries: the anterior: the posterior edge of the pubic bone, or the posterior 1–2 cm of the bladder wall (when above the superior edge of the pubic symphysis); the posterior: the anterior rectal wall, or the mesorectal fascia (when above the superior edge of the pubic symphysis); the lateral: levator ani muscles, obturator internal, sacrorectogenitopubic fascia; and the inferior: 8–12 mm below the vesicourethral anastomosis. Prior to surgery, the prostate bed is the volume occupied by the prostate and seminal vesicles. After surgery, it consists of the inferior portion of the bladder and proximal urethra, which are joined by the vesiculourethral anastomosis.
- X-ray attenuation coefficients
-
A value that characterizes how easily a material can be penetrated by X-rays, in m2/kg. Soft tissue often has the same attenuation coefficient as a tumour, so the tumour cannot readily be seen on a CT scan (which uses X-rays), unless the tumour is large (> several cm) and/or contrast material is used.
- Bone scintigraphy
-
Commonly called a bone scan, or radiolabelled diphosphonate Technetium-99m (99mTc) scan, which evaluates for bone metastases.
- Adjuvant radiation therapy
-
Post-prostatectomy radiation therapy prescribed to the prostate bed, with or without the pelvic lymph nodes, for adverse pathological features, including positive margins, extracapsular extension (pT3a) or seminal vesicle invasion (pT3b), with an undetectable PSA (historically <0.2 ng/ml).
- Surrogate end point
-
An indicator used in place of a more traditional ‘hard’ end point (such as survival), which might be more difficult, or take too long to reach to identify if a treatment works in the context of a clinical trial.
- Hard end point
-
End points including death or quality of life.
- Image-guided radiation therapy
-
An integral component of radiotherapy systems that obtains imaging coordinates of a target and/or healthy tissues before or during treatment, detects and corrects for random and systematic errors that occur in patient setup and organ motion, and increases accuracy and precision. Multiple types of image-guided radiation therapy systems exist. 2D and 3D systems detect movements interfractionally (that is between two radiotherapy sessions), novel 4D systems detect movements intrafractionally (that is during one radiotherapy session).
- Intensity-modulated radiotherapy
-
(IMRT). An advanced form of high-precision radiation that conforms the treatment volume to the shape of the tumour. The dose distribution created by IMRT is characterized by a concavity or invagination of the edge of the high doses away from the rectum, rather than a straight edge through the rectum as seen with 3D-CRT.
- Pelvic lymph node radiotherapy
-
Also called whole-pelvis radiotherapy, this is a treatment that additionally includes the following nodal regions: common iliacs, presacral (S1–3), external or internal iliacs, obturators, typically to a dose of 45–50 Gy in 2-Gy fractions. The volume generally starts at L5–S1 (although some start at L4–L5) and ends inferiorly at the femoral heads and top of the pubic symphysis.
- Gross tumour volume
-
(GTV). This is the demonstrable extent and location of the malignant growth; it consists of the macroscopic primary tumour, which for prostate cancer has historically been defined as the entire gland as well as any visualized extension into surrounding normal tissues, the regional lymph nodes, or distant metastases based on clinical data. For post-prostatectomy radiotherapy, there is typically not a measurable GTV.
- Clinical target volume
-
(CTV). This volume encompasses the gross tumour volume (GTV) as well as areas at risk of microscopic or subclinical cancer involvement. The CTV can include a margin around the prostate GTV and adjacent regions at risk of having subclinical disease. For post-prostatectomy radiotherapy, the two most common CTVs are the prostate bed ± the pelvic lymph nodes. For post-prostatectomy radiotherapy, the CTV typically does not include the GTV, as the disease is microscopic and cannot be seen on imaging.
- Planning target volume
-
(PTV). This volume encompasses the CTV plus an additional margin to account for patient movement, setup error and organ movement. For post-prostatectomy radiotherapy, this margin is typically 3–10 mm.
- α to β ratio
-
The α to β ratio describes the shape of the cell survival curve and the gradient of the two components of cell kill, α and β. The α to β ratio is used to describe the dose response of radiation on different tissues. Prostate cancer cells have a relatively low α to β ratio of 1.5, implying that those cells are more sensitive to doses delivered in larger fraction sizes. In the radiobiological linear quadratic equation, the α to β ratio is the dose at which cell killing as a result of the linear and quadratic components is equal.
- 2 Gy equivalent dose
-
(EQD2). Radiotherapy can be given with conventional fractionation (1.8–2.0 Gy per day up to ~60–70 Gy), with hypofractionation (2.1–3.0 Gy per day, up to ~50 Gy), or ultrahypofractionation/stereotactic body radiation therapy (~5–12 Gy per day, up to ~30–50 Gy). However, the overall, or isoeffective, dose is thought to be similar with all approaches, and the EQD2 helps to provide a rough estimate of this total dose if it had been delivered in 2-Gy fractions.
- Partin table
-
A mathematical formula that predicts pathological stage using retrospective prostatectomy data.
- Microscopic cell kill
-
Killing of cells that are not visible on imaging, including micrometastatic disease in the bloodstream.
Rights and permissions
About this article
Cite this article
Zaorsky, N.G., Calais, J., Fanti, S. et al. Salvage therapy for prostate cancer after radical prostatectomy. Nat Rev Urol 18, 643–668 (2021). https://doi.org/10.1038/s41585-021-00497-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00497-7
This article is cited by
-
A mitochondrion-targeted cyanine agent for NIR-II fluorescence-guided surgery combined with intraoperative photothermal therapy to reduce prostate cancer recurrence
Journal of Nanobiotechnology (2024)
-
An MRI assessment of prostate cancer local recurrence using the PI-RR system: diagnostic accuracy, inter-observer reliability among readers with variable experience, and correlation with PSA values
European Radiology (2023)
-
Current Management and Future Treatment Strategies for Patients with Metastatic Hormone-Sensitive Prostate Cancer
World Journal of Urology (2023)
-
Targeting PI3K/Akt signaling in prostate cancer therapy
Journal of Cell Communication and Signaling (2023)
-
ADT and node inclusion improves salvage radiation
Nature Reviews Urology (2022)