Abstract
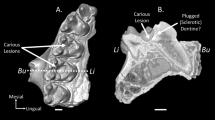
Both wild and laboratory strains of the musk shrew (Suncus murinus) have a high incidence of periodontitis. The authors completed necropsy examinations in 51 shrews to identify dental lesions including tooth loss, mobility and fractures. Dental lesions were identified in significantly more females than males, and older animals were more likely to have lesions present. Shrews with one or more dental lesions weighed significantly less than those without lesions present. Dietary supplementation with mealworms did not significantly affect the incidence of dental lesions or the body weight of male or female shrews. The authors recommend routine body weight measurement as a simple, noninvasive method of detecting shrews with an increased likelihood of having dental lesions.
This is a preview of subscription content, access via your institution
Access options



Similar content being viewed by others
References
Dryden, G.L. & Ross, J.M. Enhanced growth and development of captive musk shrews, Suncus murinus, on an improved diet. Growth 35, 311–325 (1971).
Horn, C.C., Still, L., Fitzgerald, C. & Friedman, M.I. Food restriction, refeeding, and gastric fill fail to affect emesis in musk shrews. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G25–G30 (2010).
Brame, R.E. & Lucot, J.B. Guamanian Suncus murinus responsiveness to emetic stimuli and the antiemetic effects of 8-OH-DPAT. Pharmacol. Biochem. Behav. 99, 381–384 (2011).
Matsuki, N. et al. Emesis induced by cancer chemotherapeutic agents in the Suncus murinus: a new experimental model. Jpn. J. Pharmacol. 48, 303–306 (1988).
Ueno, S., Matsuki, N. & Saito, H. Suncus murinus: a new experimental model in emesis research. Life Sci. 41, 513–518 (1987).
Ueno, S., Matsuki, N. & Saito, H. Suncus murinus as a new experimental model for motion sickness. Life Sci. 43, 413–420 (1988).
Tsutsui, C. et al. House musk shrew (Suncus murinus, order: Insectivora) as a new model animal for motilin study. Peptides 30, 318–329 (2009).
Rissman, E.F. The musk shrew, Suncus murinus, a unique animal model for the study of female behavioral endocrinology. J. Exp. Zool. Suppl. 4, 207–209 (1990).
Temple, J.L. The musk shrew (Suncus murinus): a model species for studies of nutritional regulation of reproduction. ILAR J. 45, 25–34 (2004).
Ungar, P.S. Mammal Teeth Origin, Evolution, and Diversity (Johns Hopkins University Press, Baltimore, MD, 2010).
Takata, T., Matsuura, M., Murashima, M., Miyauchi, M. & Nikai, H. Periodontitis in the house musk shrew (Suncus murinus): a potential animal model for human periodontal disease. J. Periodontol. 70, 195–200 (1999).
Yamanaka, A., Yasui, K., Sonomura, T. & Uemura, M. Development of heterodont dentition in house shrew (Suncus murinus). Eur. J. Oral Sci. 115, 433–440 (2007).
Jogahara, T., Oda, S., Kawai, T., Hanamura, H. & Koyasu, K. Numerical variation of teeth in the wild house musk shrew Suncus murinus captured from Nagasaki, Japan. Arch. Oral Biol. 53, 617–621 (2008).
Dumitrescu, A.L. Etiology and Pathogenesis of Periodontal Disease (Springer, Berlin, Germany, 2010).
Fisher, M.A., Borgnakke, W.S. & Taylor, G.W. Periodontal disease as a risk marker in coronary heart disease and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 19, 519–526 (2010).
Glickman, L.T. et al. Association between chronic azotemic kidney disease and the severity of periodontal disease in dogs. Prev. Vet. Med. 99, 193–200 (2011).
Balakrishnan, M. & Alexander, K.M. A study on aspects of feeding and food utilization of the Indian musk shrew, Suncus murinus viridescens (Blyth). Physiol. Behav. 22, 423–428 (1979).
Prakash, I. & Singh, H. Food of the shrew, Suncus murinus, inhabiting hilly tracts of south and southeastern Rajasthan. Proc. Natl. Acad. Sci. India 69, 245–250 (1999).
Advani, R. & Rana, B.D. Food of the house shrew, Suncus murinus sindensis in the Indian desert. Acta Theriologica 26, 133–134 (1981).
Kaneko, T., Iida, H., Bedford, J.M. & Mo®ri, T. Spermatozoa of the shrew, Suncus murinus, undergo the acrosome reaction and then selectively kill cells in penetrating the cumulus oophorus. Biol. Reprod. 65, 544–553 (2001).
Sakahara, S. et al. Physiological characteristics of gastric contractions and circadian gastric motility in the free-moving conscious house musk shrew (Suncus murinus). Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1106–R1113 (2010).
Andrews, P.L., Friedman, M.I., Liu, Y.L., Smith, J.E. & Sims, D.W. Potential energetic implications of emesis in the house musk shrew (Suncus murinus). Physiol Behav. 84, 519–524 (2005).
Smith, J.E., Friedman, M.I. & Andrews, P.L. Conditioned food aversion in Suncus murinus (house musk shrew)—a new model for the study of nausea in a species with an emetic reflex. Physiol. Behav. 73, 593–598 (2001).
Jogahara, T., Koyasu, K., Oda, S., Kawai, T. & Hanamura, H. Quest for the cause of oligodontia in Suncus murinus (Soricomorpha, Soricidae): Morphological re-examination. Arch. Oral Biol. 52, 836–843 (2007).
Acknowledgements
We thank Elizabeth Esken, Theresa Fennell, Amanda Furman, Teresa Garrett, Sandra Matthews, Emily Smith and Samantha Spitak for technical assistance. This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Dudley, E., Grunden, B., Crocker, C. et al. Incidence of dental lesions in musk shrews (Suncus murinus) and their association with sex, age, body weight and diet. Lab Anim 42, 422–426 (2013). https://doi.org/10.1038/laban.408
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/laban.408