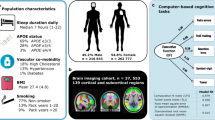
Abstract
We sought to determine which facets of sleep neurophysiology were most strongly linked to cognitive performance in 3,819 older adults from two independent cohorts, using whole-night electroencephalography. From over 150 objective sleep metrics, we identified 23 that predicted cognitive performance, and processing speed in particular, with effects that were broadly independent of gross changes in sleep quality and quantity. These metrics included rapid eye movement duration, features of the electroencephalography power spectra derived from multivariate analysis, and spindle and slow oscillation morphology and coupling. These metrics were further embedded within broader associative networks linking sleep with aging and cardiometabolic disease: individuals who, compared with similarly aged peers, had better cognitive performance tended to have profiles of sleep metrics more often seen in younger, healthier individuals. Taken together, our results point to multiple facets of sleep neurophysiology that track coherently with underlying, age-dependent determinants of cognitive and physical health trajectories in older adults.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All PSG data are freely available via the National Sleep Research Resource (http://sleepdata.org). The MESA dataset is available at https://doi.org/10.25822/n7hq-c406. The MrOS dataset is at https://doi.org/10.25822/kc27-0425. Full PSG and clinical/covariate data are available for all interested parties pending completion of a Data Access and Use Agreement and Institutional Review Board approval, as outlined on the National Sleep Research Resource website.
Code availability
Sleep EEG data were processed using the Luna package developed by S.M.P. (http://zzz.bwh.harvard.edu/luna/). The C/C++ code is available at the following GitHub repository: http://github.com/remnrem/luna-base/. Specifically, the analysis presented in this manuscript used Luna to derive measures of sleep architecture (HYPNO command) and to perform epoch-level artefact detection (SIGSTATS), signal filtering (FILTER), spectral estimation (PSD) and spindle–SO detection (SPINDLES).
Change history
16 December 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41562-020-01030-3.
References
Foley, D. J. et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep 18, 425–432 (1995).
Foley, D., Ancoli-Israel, S., Britz, P. & Walsh, J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J. Psychosom. Res. 56, 497–502 (2004).
Petersen, R. C. et al. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308 (1999).
Li, S.-C. et al. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychol. Sci. 15, 155–163 (2004).
Dijk, D. J., Duffy, J. F. & Czeisler, C. A. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol. Int. 17, 285–311 (2000).
Ohayon, M. M., Carskadon, M. A., Guilleminault, C. & Vitiello, M. V. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27, 1255–1273 (2004).
Purcell, S. M. et al. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat. Commun. 8, 15930 (2017).
Redline, S. et al. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch. Intern. Med. 164, 406–418 (2004).
Diering, G. H. et al. Homer1a drives homeostatic scaling-down of excitatory synapses during sleep. Science 355, 511–515 (2017).
Wilson, M. A. & McNaughton, B. L. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994).
Stickgold, R. & Walker, M. P. Sleep-dependent memory consolidation and reconsolidation. Sleep Med. 8, 331–343 (2007).
De Vivo, L. et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355, 507–510 (2017).
Scullin, M. K. & Bliwise, D. L. Sleep, cognition, and normal aging: integrating a half century of multidisciplinary research. Perspect. Psychol. Sci. 10, 97–137 (2015).
Ohayon, M. M. & Vecchierini, M.-F. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep 28, 981–989 (2005).
Keage, H. A. D. et al. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 13, 886–892 (2012).
Song, Y. et al. Relationships between sleep stages and changes in cognitive function in older men: the MrOS Sleep Study. Sleep 38, 411–421 (2015).
Cavuoto, M. G. et al. Objective but not subjective sleep predicts memory in community-dwelling older adults. J. Sleep Res. 25, 475–485 (2016).
Blackwell, T. et al. Associations between sleep architecture and sleep-disordered breathing and cognition in older community-dwelling men: the Osteoporotic Fractures in Men sleep study. J. Am. Geriatr. Soc. 59, 2217–2225 (2011).
Spira, A. P. et al. Actigraphic sleep duration and fragmentation in older women: associations with performance across cognitive domains. Sleep 40, zsx073 (2017).
Devore, E. E. et al. Sleep duration in midlife and later life in relation to cognition. J. Am. Geriatr. Soc. 62, 1073–1081 (2014).
Ramos, A. R. et al. Sleep duration and neurocognitive function in the Hispanic Community Health Study/Study of Latinos. Sleep 39, 1843–1851 (2016).
Blackwell, T. et al. Association of sleep characteristics and cognition in older community-dwelling men: the MrOS Sleep Study. Sleep 34, 1347–1356 (2011).
Potvin, O. et al. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep 35, 491–499 (2012).
Jaussent, I. et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep 35, 1201–1207 (2012).
Foley, D. et al. Daytime sleepiness is associated with 3-year incident dementia and cognitive decline in older Japanese–American men. J. Am. Geriatr. Soc. 49, 1628–1632 (2001).
Carvalho, D. Z. et al. Association of excessive daytime sleepiness with longitudinal β-amyloid accumulation in elderly persons without dementia. JAMA Neurol. 75, 672–680 (2018).
Mander, B. A., Winer, J. R., Jagust, W. J. & Walker, M. P. Sleep: a novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 39, 552–566 (2016).
Lafortune, M. et al. Sleep spindles and rapid eye movement sleep as predictors of next morning cognitive performance in healthy middle-aged and older participants. J. Sleep Res. 23, 159–167 (2014).
Helfrich, R. F., Mander, B. A., Jagust, W. J., Knight, R. T. & Walker, M. P. Old brains come uncoupled in sleep: slow wave–spindle synchrony, brain atrophy, and forgetting. Neuron 97, 221–230.e4 (2018).
Winer, J. R. et al. Sleep as a potential biomarker of tau and β-amyloid burden in the human brain. J. Neurosci. 39, 6315–6324 (2019).
Muehlroth, B. E. et al. Precise slow oscillation–spindle coupling promotes memory consolidation in younger and older adults. Sci. Rep. 9, 1940 (2019).
Prinz, P. N. et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol. Aging 3, 361–370 (1982).
Pase, M. P. et al. Sleep architecture and the risk of incident dementia in the community. Neurology 89, 1244–1250 (2017).
Varga, A. W. et al. Apnea-induced rapid eye movement sleep disruption impairs human spatial navigational memory. J. Neurosci. 34, 14571–14577 (2014).
Bjorness, T. E., Riley, B. T., Tysor, M. K. & Poe, G. R. REM restriction persistently alters strategy used to solve a spatial task. Learn. Mem. 12, 352–359 (2005).
Smith, C. & Rose, G. M. Evidence for a paradoxical sleep window for place learning in the Morris water maze. Physiol. Behav. 59, 93–97 (1996).
Yaffe, K. et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. J. Am. Med. Assoc. 306, 613–619 (2011).
Burke, G., Lima, J., Wong, N. D. & Narula, J. The multiethnic study of atherosclerosis. Glob. Heart 11, 267–268 (2016).
Bild, D. E. et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am. J. Epidemiol. 156, 871–881 (2002).
Chen, X. et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 38, 877–888 (2015).
Wechsler, D. Adult Intelligence Scale-III (WAIS-III) (Psychological Corporation/Harcourt, 1996).
Teng, E. L. et al. The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int. Psychogeriatr. 6, 45–58 (1994).
Fitzpatrick, A. L. et al. Sociodemographic correlates of cognition in the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Geriatr. Psychiatry 23, 684–697 (2015).
Reitan, R. M. Validity of the trail making test as an indicator of organic brain damage. Percept. Mot. Skills 8, 271–276 (1958).
Teng, E. L. & Chui, H. C. The Modified Mini-Mental State (3MS) examination. J. Clin. Psychiatry 48, 314–318 (1987).
Kelland, D. Z. & Lewis, R. F. The Digit Vigilance Test: reliability, validity, and sensitivity to diazepam. Arch. Clin. Neuropsychol. 11, 339–344 (1996).
Cappuccio, F. P. & Miller, M. A. Sleep and cardio-metabolic disease. Curr. Cardiol. Rep. 19, 110 (2017).
Köhler, S. et al. Temporal evolution of cognitive changes in incident hypertension: prospective cohort study across the adult age span. Hypertension 63, 245–251 (2014).
Roberts, R. E. & Duong, H. T. The prospective association between sleep deprivation and depression among adolescents. Sleep 37, 239–244 (2014).
Byers, A. L. & Yaffe, K. Depression and risk of developing dementia. Nat. Rev. Neurol. 7, 323–331 (2011).
Kluge, M., Schüssler, P. & Steiger, A. Duloxetine increases stage 3 sleep and suppresses rapid eye movement (REM) sleep in patients with major depression. Eur. Neuropsychopharmacol. 17, 527–531 (2007).
DeMartinis, N. A. & Winokur, A. Effects of psychiatric medications on sleep and sleep disorders. CNS Neurol. Disord. Drug Targets 6, 17–29 (2007).
Scheer, F. A. J. L. et al. Repeated melatonin supplementation improves sleep in hypertensive patients treated with beta-blockers: a randomized controlled trial. Sleep 35, 1395–1402 (2012).
Laventure, S. et al. Beyond spindles: interactions between sleep spindles and boundary frequencies during cued reactivation of motor memory representations. Sleep 41, zsy142 (2018).
Dubé, J. et al. Cortical thinning explains changes in sleep slow waves during adulthood. J. Neurosci. 35, 7795–7807 (2015).
Carrier, J. et al. Sleep slow wave changes during the middle years of life. Eur. J. Neurosci. 33, 758–766 (2011).
Martin, N. et al. Topography of age-related changes in sleep spindles. Neurobiol. Aging 34, 468–476 (2013).
Jackson, C. L., Patel, S. R., Jackson, W. B., Lutsey, P. L. & Redline, S.Agreement between self-reported and objectively measured sleep duration among white, Black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep 41, zsy057 (2018).
Bianchi, M. T., Thomas, R. J. & Westover, M. B. An open request to epidemiologists: please stop querying self-reported sleep duration. Sleep Med. 35, 92–93 (2017).
Johnson, D. A. et al. Greater cognitive deficits with sleep-disordered breathing among individuals with genetic susceptibility to Alzheimer disease. The Multi-Ethnic Study of Atherosclerosis. Ann. Am. Thorac. Soc. 14, 1697–1705 (2017).
Liu, Y. et al. Methylomics of gene expression in human monocytes. Hum. Mol. Genet. 22, 5065–5074 (2013).
Sun, H. et al. Brain age from the electroencephalogram of sleep. Neurobiol. Aging 74, 112–120 (2019).
Jorm, A. F., Masaki, K. H., Petrovitch, H., Ross, G. W. & White, L. R. Cognitive deficits 3 to 6 years before dementia onset in a population sample: the Honolulu–Asia Aging Study. J. Am. Geriatr. Soc. 53, 452–455 (2005).
Brody, J. A. & Schneider, E. L. Diseases and disorders of aging: an hypothesis. J. Chronic Dis. 39, 871–876 (1986).
Toepper, M. Dissociating normal aging from Alzheimer’s disease: a view from cognitive neuroscience. J. Alzheimers Dis. 57, 331–352 (2017).
Wang, J. L. et al. Suprachiasmatic neuron numbers and rest–activity circadian rhythms in older humans. Ann. Neurol. 78, 317–322 (2015).
Swaab, D. F., Fliers, E. & Partiman, T. S. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 342, 37–44 (1985).
Blackwell, T. et al. Poor sleep is associated with impaired cognitive function in older women: the Study of Osteoporotic Fractures. J. Gerontol. A Biol. Sci. Med. Sci. 61, 405–410 (2006).
Lim, A. S. P. et al. Sleep is related to neuron numbers in the ventrolateral preoptic/intermediate nucleus in older adults with and without Alzheimer’s disease. Brain 137, 2847–2861 (2014).
Ward, A. M. et al. Daytime sleepiness is associated with decreased default mode network connectivity in both young and cognitively intact elderly subjects. Sleep 36, 1609–1615 (2013).
Carvalho, D. Z. et al. Excessive daytime sleepiness and fatigue may indicate accelerated brain aging in cognitively normal late middle-aged and older adults. Sleep Med. 32, 236–243 (2017).
Knutson, K. L. & Turek, F. W. The U-shaped association between sleep and health: the 2 peaks do not mean the same thing. Sleep 29, 878–879 (2006).
Patel, S. R., Malhotra, A., Gottlieb, D. J., White, D. P. & Hu, F. B. Correlates of long sleep duration. Sleep 29, 881–889 (2006).
Grandner, M. A. & Drummond, S. P. A. Who are the long sleepers? Towards an understanding of the mortality relationship. Sleep Med. Rev. 11, 341–360 (2007).
Rauchs, G. et al. Is there a link between sleep changes and memory in Alzheimer’s disease? NeuroReport 19, 1159–1162 (2008).
Mölle, M., Bergmann, T. O., Marshall, L. & Born, J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep 34, 1411–1421 (2011).
Feld, G. & Diekelmann, S.Sleep smart—optimizing sleep for declarative learning and memory. Front. Psychol. 6, 622 (2015).
Huber, R., Ghilardi, M. F., Massimini, M. & Tononi, G. Local sleep and learning. Nature 430, 78–81 (2004).
Ju, Y.-E. S. et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain 140, 2104–2111 (2017).
Mander, B. A. et al. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat. Neurosci. 18, 1051–1057 (2015).
Wang, C. & Holtzman, D. M. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 45, 104–120 (2020).
Feinberg, I., Koresko, R. L. & Heller, N. EEG sleep patterns as a function of normal and pathological aging in man. J. Psychiatr. Res. 5, 107–144 (1967).
Spiegel, R., Herzog, A. & Köberle, S. Polygraphic sleep criteria as predictors of successful aging: an exploratory longitudinal study. Biol. Psychiatry 45, 435–442 (1999).
Kim, S. J., Lee, J. H., Lee, D. Y., Jhoo, J. H. & Woo, J. I. Neurocognitive dysfunction associated with sleep quality and sleep apnea in patients with mild cognitive impairment. Am. J. Geriatr. Psychiatry 19, 374–381 (2011).
Della Monica, C., Johnsen, S., Atzori, G., Groeger, J. A. & Dijk, D.-J. Rapid eye movement sleep, sleep continuity and slow wave sleep as predictors of cognition, mood, and subjective sleep quality in healthy men and women, aged 20–84 years. Front. Psychiatry 9, 255 (2018).
Markowska, A. L. et al. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol. Aging 10, 31–43 (1989).
Stone, W. S., Altman, H. J., Berman, R. F., Caldwell, D. F. & Kilbey, M. M. Association of sleep parameters and memory in intact old rats and young rats with lesions in the nucleus basalis magnocellularis. Behav. Neurosci. 103, 755–764 (1989).
Liguori, C. et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 71, 1498–1505 (2014).
Czeisler, C. A., Zimmerman, J. C., Ronda, J. M., Moore-Ede, M. C. & Weitzman, E. D. Timing of REM sleep is coupled to the circadian rhythm of body temperature in man. Sleep 2, 329–346 (1980).
Roh, J. H. et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J. Exp. Med. 211, 2487–2496 (2014).
Astori, S., Wimmer, R. D. & Lüthi, A. Manipulating sleep spindles—expanding views on sleep, memory, and disease. Trends Neurosci. 36, 738–748 (2013).
Marshall, L., Helgadóttir, H., Mölle, M. & Born, J. Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613 (2006).
Wilckens, K. A., Ferrarelli, F., Walker, M. P. & Buysse, D. J. Slow-wave activity enhancement to improve cognition. Trends Neurosci. 41, 470–482 (2018).
Bellesi, M., Riedner, B. A., Garcia-Molina, G. N., Cirelli, C. & Tononi, G. Enhancement of sleep slow waves: underlying mechanisms and practical consequences. Front. Syst. Neurosci. 8, 208 (2014).
Papalambros, N. A. et al. Acoustic enhancement of sleep slow oscillations and concomitant memory improvement in older adults. Front. Hum. Neurosci. 11, 109 (2017).
Johns, M. W. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 14, 540–545 (1991).
Levine, D. W. et al. Validation of the Women’s Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosom. Med. 67, 98–104 (2005).
Horne, J. A. & Ostberg, O. A self-assessment questionnaire to determine morningness–eveningness in human circadian rhythms. Int. J. Chronobiol. 4, 97–110 (1976).
Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 28, 193–213 (1989).
Weaver, T. E. et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 20, 835–843 (1997).
Rechtschaffen, A. & Kales, A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects (United States Government Printing Office, 1968).
Feinberg, I. & Floyd, T. C. Systematic trends across the night in human sleep cycles. Psychophysiology 16, 283–291 (1979).
Hjorth, B. EEG analysis based on time domain properties. Electroencephalogr. Clin. Neurophysiol. 29, 306–310 (1970).
Tenke, C. E. & Kayser, J. Reference-free quantification of EEG spectra: combining current source density (CSD) and frequency principal components analysis (fPCA). Clin. Neurophysiol. 116, 2826–2846 (2005).
Miller, K. J. et al. Human motor cortical activity is selectively phase-entrained on underlying rhythms. PLoS Comput. Biol. 8, e1002655 (2012).
Duffy, F. H. & Als, H. A stable pattern of EEG spectral coherence distinguishes children with autism from neuro-typical controls—a large case control study. BMC Med. 10, 64 (2012).
Otsu, N. A threshold selection method from gray-level histograms. IEEE Trans. Syst. Man Cybern. 9, 62–66 (1979).
Ayoub, A. et al. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity. Sleep 36, 905–911 (2013).
Zeitlhofer, J. et al. Topographic distribution of sleep spindles in young healthy subjects. J. Sleep Res. 6, 149–155 (1997).
De Gennaro, L. & Ferrara, M. Sleep spindles: an overview. Sleep Med. Rev. 7, 423–440 (2003).
Massimini, M., Huber, R., Ferrarelli, F., Hill, S. & Tononi, G. The sleep slow oscillation as a traveling wave. J. Neurosci. 24, 6862–6870 (2004).
Dang-Vu, T. T. et al. Spontaneous neural activity during human slow wave sleep. Proc. Natl Acad. Sci. USA 105, 15160–15165 (2008).
Muehlroth, B. E. & Werkle-Bergner, M. Understanding the interplay of sleep and aging: methodological challenges. Psychophysiology 57, e13523 (2020).
Cohen, M. X. Analyzing Neural Time Series Data: Theory and Practice (The MIT Press, 2014).
McInnes, L., Healy, J. & Melville, J. UMAP: uniform manifold approximation and projection for dimension reduction. Preprint at https://arxiv.org/abs/1802.03426 (2018).
Campello, R. J. G. B., Moulavi, D. & Sander, J. in Advances in Knowledge Discovery and Data Mining Vol. 7819 (eds Pei, J. et al.) 160–172 (Springer, 2013).
Acknowledgements
MESA and the MESA SNP Health Association Resource project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the NHLBI, and by grants UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1-TR-001881, DK063491, K24 AG045334, P30AG059303 and R01AG058969. Funding support for the sleep PSG dataset was provided by grant HL56984. Funding for SNP Health Association Resource genotyping was provided by NHLBI contract N02-HL-64278. Genotyping was performed at Affymetrix (Santa Clara, California, United States) and the Broad Institute of Harvard and MIT (Boston, Massachusetts, United States) using the Affymetrix Genome-Wide Human SNP Array 6.0. The authors thank the other investigators, the staff and the participants of the MESA study for valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The MrOS is supported by National Institutes of Health (NIH) funding. The National Institute on Aging (NIA), National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Center for Advancing Translational Sciences (NCATS) and NIH Roadmap for Medical Research provide support under the following grant numbers: U01 AG027810; U01 AG042124; U01 AG042139; U01 AG042140; U01 AG042143; U01 AG042145; U01 AG042168; U01 AR066160; and UL1 TR000128. The NHLBI provides funding for the MrOS Sleep ancillary study ‘Outcomes of Sleep Disorders in Older Men’ under the following grant numbers: R01 HL071194; R01 HL070848; R01 HL070847; R01 HL070842; R01 HL070841; R01 HL070837; R01 HL070838; and R01 HL070839. In addition, this work was also supported by NIH/National Institute of Mental Health grant R03 MH108908 (to S.M.P.), NIH/NHLBI grant R01 HL146339 (to S.M.P.), NIH/NHLBI grant R21 HL145492 (to S.M.P.), NIH/National Institute on Minority Health and Health Disparities grant R21 MD012738 (to S.M.P.), NIH grant K23AG049955 (to J.M.D.), NIH/NHLBI grant K01HL138211 (to D.J.), NIH/NHLBI grant R35 HL135818 (to S.R.), NIH/National Institute of Neurological Disorders and Stroke grant R01 NS096177 (to M.J.P.), NIH/National Institute on Aging grant R01 AG054081 (to M.J.P.), K24 AG045334 (to J.A.L.), a Beth Israel Deaconess Medical Center Neurology Department Grant (to I.D.) and NIH/NHLBI grant R24 HL114473 (to S.R., S.M. and S.M.P.). This work is, in part, also a publication of the US Department of Agriculture/Agricultural Research Service (USDA/ADS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine (Houston, Texas), funded in part by the USDA/ADS (cooperative agreement 58-3092-5-001). The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products or organizations imply endorsement from the US Government. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
I.D., S.R. and S.M.P. conceived of and planned the study. S.R.R., A.L.F., A.C.W., T.S., H.T.N., J.A.L. and S.R. collected the primary sleep and cognitive data in MESA. K.L.S., G.J.T., K.Y. and S.R. collected the primary sleep and cognitive data in MrOS. S.M.P. developed the analytical software and approach. I.D., S.M., M.J.P., V.M.G.T.H.V.D.K., D.J., J.M.D. and K.E.B. discussed the analytical approach/results. S.M.P., I.D. and S.R. drafted the manuscript. All authors reviewed and commented on the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Primary Handling Editor: Marike Schiffer.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–18, Supplementary Tables 1–7, Supplementary Methods and Supplementary References.
Supplementary Data 1–4
Supplementary Data 1. All baseline model results for MESA. Supplementary Data 2. All baseline model results for MrOS. Supplementary Data 3. SO, spindle/SO coupling and spindle/SWA coupling results under alternate SO definitions in MESA. Supplementary Data 4. SO, spindle/SO coupling and spindle/SWA coupling results under alternate SO definitions in MrOS.
Rights and permissions
About this article
Cite this article
Djonlagic, I., Mariani, S., Fitzpatrick, A.L. et al. Macro and micro sleep architecture and cognitive performance in older adults. Nat Hum Behav 5, 123–145 (2021). https://doi.org/10.1038/s41562-020-00964-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41562-020-00964-y
This article is cited by
-
Sleep alterations as a function of 88 health indicators
BMC Medicine (2024)
-
Attention and executive function impairments in obstructive sleep apnea are associated with decreased sleep spindles
Acta Neurologica Belgica (2024)
-
The Association of Upper Airway Anatomy with Brain Structure: The Multi-Ethnic Study of Atherosclerosis
Brain Imaging and Behavior (2024)
-
The association of upper airway anatomy with cognitive test performance: the Multi-Ethnic Study of Atherosclerosis
BMC Neurology (2023)
-
Decoding information about cognitive health from the brainwaves of sleep
Scientific Reports (2023)