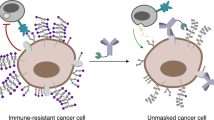
Abstract
Because of the blood–tumour barrier and cross-reactivity with healthy tissues, immune checkpoint blockade therapy against glioblastoma has inadequate efficacy and is associated with a high risk of immune-related adverse events. Here we show that anti-programmed death-ligand 1 antibodies conjugated with multiple poly(ethylene glycol) (PEG) chains functionalized to target glucose transporter 1 (which is overexpressed in brain capillaries) and detaching in the reductive tumour microenvironment augment the potency and safety of checkpoint blockade therapy against glioblastoma. In mice bearing orthotopic glioblastoma tumours, a single dose of glucosylated and multi-PEGylated antibodies reinvigorated antitumour immune responses, induced immunological memory that protected the animals against rechallenge with tumour cells, and suppressed autoimmune responses in the animals’ healthy tissues. Drug-delivery formulations leveraging multivalent ligand interactions and the properties of the tumour microenvironment to facilitate the crossing of blood–tumour barriers and increase drug specificity may enhance the efficacy and safety of other antibody-based therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw and analysed datasets generated during the study are too large to be publicly shared, but they are available for research purposes from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
He, M. et al. Immune checkpoint inhibitor-based strategies for synergistic cancer therapy. Adv. Healthc. Mater. 10, 2002104 (2021).
Aldape, K. et al. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 16, 509–520 (2019).
Lim, M., Xia, Y., Bettegowda, C. & Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 15, 422–442 (2018).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Arvanitis, C. D., Ferraro, G. B. & Jain, R. K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 20, 26–41 (2020).
Trang, V. H. et al. A coiled-coil masking domain for selective activation of therapeutic antibodies. Nat. Biotechnol. 37, 761–765 (2019).
Naidoo, J. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 26, 2375–2391 (2015).
Sharpe, A. H., Wherry, E. J., Ahmed, R. & Freeman, G. J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 8, 239–245 (2007).
Ramos-Casals, M. et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 6, 38 (2020).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16, 563–580 (2019).
Brahmer, J. R. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 36, 1714–1768 (2018).
Abdel-Wahab, N., Shah, M., Lopez-Olivo, M. A. & Suarez-Almazor, M. E. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease. Ann. Intern. Med. 168, 121–130 (2018).
Gan, H. K., van den Bent, M., Lassman, A. B., Reardon, D. A. & Scott, A. M. Antibody–drug conjugates in glioblastoma therapy: the right drugs to the right cells. Nat. Rev. Clin. Oncol. 14, 695–707 (2017).
Desnoyers, L. R. et al. Tumor-specific activation of an EGFR-targeting probody enhances therapeutic index. Sci. Transl. Med. 5, 207ra144–207ra144 (2013).
Erster, O. et al. Site-specific targeting of antibody activity in vivo mediated by disease-associated proteases. J. Control. Release 161, 804–812 (2012).
Yang, Y. et al. Preclinical studies of a Pro-antibody-drug conjugate designed to selectively target EGFR-overexpressing tumors with improved therapeutic efficacy. MAbs 8, 405–413 (2016).
Yang, Y. et al. Generation and characterization of a target-selectively activated antibody against epidermal growth factor receptor with enhanced anti-tumor potency. MAbs 7, 440–450 (2015).
Mi, P., Cabral, H. & Kataoka, K. Ligand-installed nanocarriers toward precision therapy. Adv. Mater. 32, 1902604 (2020).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Ricci-Vitiani, L. et al. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 468, 824–828 (2010).
Francis, J. M. et al. EGFR variant heterogeneity in glioblastoma resolved through single-nucleus sequencing. Cancer Discov. 4, 956–971 (2014).
Roberts-Rapp, L. et al. 28PD identifying the correct patient (pt) population for ABT-414: biomarker assays for epidermal growth factor receptor (EGFR) in pts with glioblastoma (GBM). Ann. Oncol. 26, ix8–ix15 (2015).
Anraku, Y. et al. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat. Commun. 8, 1001 (2017).
Suzuki, K. et al. Glucose transporter 1-mediated vascular translocation of nanomedicines enhances accumulation and efficacy in solid tumors. J. Control. Release 301, 28–41 (2019).
Agnihotri, S. & Zadeh, G. Metabolic reprogramming in glioblastoma: the influence of cancer metabolism on epigenetics and unanswered questions. Neuro Oncol. 18, 160–172 (2015).
Hao, C. et al. PD-L1 expression in glioblastoma, the clinical and prognostic significance: a systematic literature review and meta-analysis. Front. Oncol. 10, 1015 (2020).
Chen, R. Q., Liu, F., Qiu, X. Y. & Chen, X. Q. The prognostic and therapeutic value of PD-L1 in glioma. Front. Pharmacol. 9, 1503 (2019).
Berghoff, A. S. et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 17, 1064–1075 (2014).
Liu, Y. et al. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. J. Neurosci. 33, 14231–14245 (2013).
Chang, C.-H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Backos, D. S., Franklin, C. C. & Reigan, P. The role of glutathione in brain tumor drug resistance. Biochem. Pharmacol. 83, 1005–1012 (2012).
Gamcsik, M. P., Kasibhatla, M. S., Teeter, S. D. & Colvin, O. M. Glutathione levels in human tumors. Biomarkers 17, 671–691 (2012).
Greenfield, N. J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 1, 2876–2890 (2006).
Louis-Jeune, C., Andrade-Navarro, M. A. & Perez-Iratxeta, C. Prediction of protein secondary structure from circular dichroism using theoretically derived spectra. Proteins 80, 374–381 (2012).
Wang, D. et al. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci. Immunol. 4, eaau6584 (2019).
Zhu, A. et al. Dually pH/reduction-responsive vesicles for ultrahigh-contrast fluorescence imaging and thermo-chemotherapy-synergized tumor ablation. ACS Nano 9, 7874–7885 (2015).
Wainwright, D. A. et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4, and PD-L1 in mice with brain tumors. Clin. Cancer Res. 20, 5290–5301 (2014).
Harris, J. M. & Chess, R. B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2, 214–221 (2003).
Thorens, B. & Mueckler, M. Glucose transporters in the 21st Century. Am. J. Physiol. 298, E141–E145 (2010).
Cheng, F. & Eng, C. PTEN mutations trigger resistance to immunotherapy. Trends Mol. Med. 25, 461–463 (2019).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
Dangaj, D. et al. Cooperation between constitutive and inducible chemokines enables T Cell engraftment and immune attack in solid tumors. Cancer Cell 35, 885–900.e810 (2019).
Chow, M. T. et al. Intratumoral activity of the CXCR3 chemokine system is required for the efficacy of anti-PD-1 therapy. Immunity 50, 1498–1512.e1495 (2019).
McKelvey, K. J. et al. Temporal and spatial modulation of the tumor and systemic immune response in the murine Gl261 glioma model. PLoS ONE 15, e0226444 (2020).
Zhang, P. et al. Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma. Proc. Natl Acad. Sci. USA 116, 23714–23723 (2019).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Zhao, J. et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 25, 462–469 (2019).
Mazanet, M. M. & Hughes, C. C. W. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J. Immunol. 169, 3581–3588 (2002).
Ma, Q. et al. Calming cytokine storm in pneumonia by targeted delivery of TPCA-1 using platelet-derived extracellular vesicles. Matter 3, 287–301 (2020).
Kaufman, H. L. et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 17, 1374–1385 (2016).
Gulley, J. L. et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 18, 599–610 (2017).
Gan, H. K., Burgess, A. W., Clayton, A. H. A. & Scott, A. M. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 72, 2924–2930 (2012).
Gan, H. K. et al. A phase I and biodistribution study of ABT-806i, an 111indium-labeled conjugate of the tumor-specific anti-EGFR antibody ABT-806. J. Clin. Oncol. 31, 2520 (2013).
Bezu, L. et al. Combinatorial strategies for the induction of immunogenic cell death. Front. Immunol. 6, 187 (2015).
Kinoh, H. et al. Translational nanomedicine boosts anti-PD1 therapy to eradicate orthotopic PTEN-negative glioblastoma. ACS Nano 14, 10127–10140 (2020).
Alconcel, S. N. S., Baas, A. S. & Maynard, H. D. FDA-approved poly(ethylene glycol)-protein conjugate drugs. Polym. Chem. 2, 1442–1448 (2011).
Chan, H. Y., Choi, J., Jackson, C. & Lim, M. Combination immunotherapy strategies for glioblastoma. J. Neuro Oncol. 151, 375–391 (2021).
LeBlanc, A. K. et al. Creation of an NCI comparative brain tumor consortium: informing the translation of new knowledge from canine to human brain tumor patients. Neuro Oncol. 18, 1209–1218 (2016).
Acknowledgements
We thank Y. Tezuka and S. Fukushima for the technical assistance. This work was supported by the Center of Innovation Science and Technology-based Radical Innovation and Entrepreneurship Program (COI STREAM) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), JSPS KAKENHI Grant-in-Aid for Scientific Research (A) (JP18H04170) and Project for Cancer Research and Therapeutic Evolution (P-CREATE) (JP19cm0106202) from Japan Agency for Medical Research and Development (AMED).
Author information
Authors and Affiliations
Contributions
T.Y. conducted the synthesis, characterization, cell studies, animal experiments and data analysis. X.L. helped in the construction of the orthotopic brain tumour model and animal experiments. Y.M. helped in the CD spectra test and antitumour immune response analysis. H.Z., J.X. and H.K. contributed to the FACS experiments and data analysis. Y.A. helped in the analysis and discussion on the heterogeneity of GLUT1 in the brain tumours. T.Y. and K.K. conceived the idea and designed the experiments. T.Y. and H.C. discussed the data and wrote the manuscript. K.K. commented on and revised the manuscript, and supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Biomedical Engineering thanks Esther Chang, Dai Fukumura and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Spatial analysis of native aPD-L1 and Gluc-S-aPD-L1 distribution in GL261 tumours.
a,b, Fluorescent images of the distribution of Alexa Fluor 647-labelled (a) native aPD-L1 and (b) Gluc0-S-aPD-L1 (cyan) in GL261 tumour sections stained with anti-CD31 antibody (red), anti-GLUT1 antibody (green) and DAPI (blue), white dash line indicated tumour boundary. Scale bar, 1,000 μm. c, Fluorescence intensity of native aPD-L1, Gluc0-S-aPD-L1 and Gluc25-S-aPD-L1 at tumour region at 24 h post-injection. d, Calculated fluorescence intensity ratio of tumour to normal brain in native aPD-L1, Gluc0-S-aPD-L1 and Gluc25-S-aPD-L1 groups at 24 h post-injection. Column heights and error bars represent means ± s.e.m. (n = 3).
Extended Data Fig. 2 Distribution and quantitative analysis of Gluc25-S-aPD-L1 in GL261 tumour regions with high and low GLUT1 levels.
a,b, Fluorescence images of GLUT1-rich areas (a) and GLUT1-low areas (b) in GL261 tumour sections stained with Alexa Fluor 647-labelled Gluc25-S-aPD-L1 (cyan), anti-CD31 antibody (red), anti-GLUT1 antibody (green) and DAPI (blue). 6 GLUT1-rich areas and 6 GLUT1-low areas from sections of 3 different tumours were selected. Scale bar, 100 μm. c, Blood vessel density and GLUT1 density in GLUT1-rich areas and GLUT1-low areas. d, Co-localization rate of blood vessel and GLUT1 in GLUT1-rich areas and GLUT1-low areas. e, Fluorescence intensity of Alexa Fluor 647-labelled Gluc25-S-aPD-L1 in GLUT1-rich areas and GLUT1-low areas. Data are means ± s.e.m. (n = 6). Statistical significance was calculated by student’s t-test.
Extended Data Fig. 3 In vivo antitumour efficacy of Gluc25-S-aPD-L1 in CT2A tumour models.
a, Timeline schedule for the treatment of CT2A tumour models. b, Bioluminescence images of CT2A tumour-bearing mice treated with saline, native aPD-L1, and Gluc25-S-aPD-L1 (aPD-L1: 1.5 mg per kg, n = 5). c, Region-of-interest analysis of bioluminescence intensities from whole brain. d, Survival curves for treated and control mice (n = 5). Statistical significance was calculated by log-rank test.
Extended Data Fig. 4 Quantitative analysis of chemoattractants production in GL261 tumour after various treatments.
a,b, Levels of chemoattractants such as Cxcl9 (a) and Cxcl10 (b) inside GL261 tumour of mice at 3 d post-injection of saline, native aPD-L1, Gluc0-S-aPD-L1 and Gluc25-S-aPD-L1 at the dose of 1.5 mg kg−1 aPD-L1 (n = 3 biologically independent samples). Data are presented as means ± s.e.m. Statistical significance was calculated by one-way analysis of variance (ANOVA) with Tukey’s post hoc test.
Supplementary information
Source data
Source data for Fig. 3c
Statistical source data.
Source data for Extended Data Fig. 3c
Statistical source data.
Rights and permissions
About this article
Cite this article
Yang, T., Mochida, Y., Liu, X. et al. Conjugation of glucosylated polymer chains to checkpoint blockade antibodies augments their efficacy and specificity for glioblastoma. Nat Biomed Eng 5, 1274–1287 (2021). https://doi.org/10.1038/s41551-021-00803-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-021-00803-z
This article is cited by
-
Nanoparticle-mediated TRPV1 channel blockade amplifies cancer thermo-immunotherapy via heat shock factor 1 modulation
Nature Communications (2023)
-
Controlling the biodistribution and clearance of nanomedicines
Nature Reviews Bioengineering (2023)
-
Engineered nanomedicines block the PD-1/PD-L1 axis for potentiated cancer immunotherapy
Acta Pharmacologica Sinica (2022)