Abstract
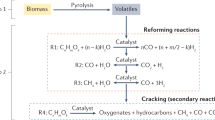
Hydrogen is playing an increasingly larger role in clean energy technologies and the emerging hydrogen economy. However, efficient and selective H2 production from renewable resources is rare so far. Herein, we describe a dehydrogenation route that is applicable to various kinds of non-food-related biomass and daily waste, such as wheat straw, corn straw, rice straw, reed, bagasse, bamboo sawdust, cardboard and newspaper. H2 yields up to 95% were achieved by a one-pot, two-step reaction with a 69 ppm molecularly defined iridium catalyst bearing an imidazoline moiety from formic acid, which was in turn obtained via a 1 v% dimethyl sulfoxide-promoted hydrolysis–oxidation of biomass. Formation of the unwanted side products CO and CH4 was no more than 22 and 2 ppm, respectively, while CO2 was captured as carbonate. The resulting hydrogen gas can be directly applied in proton exchange membrane fuel cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
09 August 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
05 August 2019
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
30 July 2018
In the version of this Article originally published, there were several errors in Table 1: in structure 1, the NH group bonded to the Ir should have been a H atom; the NR2 group bonded to the Ir should have been NH; in structures 2, 4 and 5, the OH groups bonded to the Ir atoms should have been OH2 groups; in structure 6 the OH2 group bonded to Ir was erroneously bonded with the below N; and in structure 10, the N=N bond should have been N–N. In structure 12 of Fig. 4, the CH2 between the two oxygen atoms should have been a CH group. Finally, the affiliations within the Supplementary Information were not consistent with those of the main text, a new Supplementary Information file has been uploaded.
References
Nikolaidis, P. & Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sust. Energ. Rev. 67, 597–611 (2017).
Stolten, D. (ed.) Hydrogen and Fuel Cells (Wiley-VCH, Weinheim, 2010).
Turner, J. A. Sustainable hydrogen production. Science 305, 972–974 (2004).
Muradov, N. Z. & Veziroğlu, T. N. “Green” path from fossil-based to hydrogen economy: an overview of carbon-neutral technologies. Int. J. Hydrogen Energy 33, 6804–6839 (2008).
Sanderson, K. Lignocellulose: a chewy problem. Nature 474, S12–S14 (2011).
Cortright, R. D., Davda, R. & Dumesic, J. A. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature 418, 964–967 (2002).
Huber, G. W., Iborra, S. & Corma, A. Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 106, 4044–4098 (2006).
Sikarwar, V. S. et al. An overview of advances in biomass gasification. Energy Environ. Sci. 9, 2939–2977 (2016).
Kan, T., Strezov, V. & Evans, T. J. Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Renew. Sust. Energ. Rev. 57, 1126–1140 (2016).
Reddy, S. N., Nanda, S., Dalai, A. K. & Kozinski, J. A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 39, 6912–6926 (2014).
Stonor, M. R., Ferguson, T. E., Chen, J. G. & Park, A.-H. A. Biomass conversion to H2 with substantially suppressed CO2 formation in the presence of group I & group II hydroxides and a Ni/ZrO2 catalyst. Energy Environ. Sci. 8, 1702–1706 (2015).
Woodward, J. et al. In vitro hydrogen production by glucose dehydrogenase and hydrogenase. Nat. Biotechnol. 14, 872–874 (1996).
Jonathan, W., Orr, M., Cordray, K. & Greenbaum, E. Biotechnology: enzymatic production of biohydrogen. Nature 405, 1014–1015 (2000).
Rollin, J. A. et al. High-yield hydrogen production from biomass by in vitro metabolic engineering: mixed sugars coutilization and kinetic modeling. Proc. Natl Acad. Sci. USA 112, 4964–4969 (2015).
Xia, A. et al. Fermentative hydrogen production using algal biomass as feedstock. Renew. Sust. Energ. Rev. 51, 209–230 (2015).
Liu, W. et al. High efficiency hydrogen evolution from native biomass electrolysis. Energy Environ. Sci. 9, 467–472 (2016).
Kadier, A. et al. Recent advances and emerging challenges in microbial electrolysis cells (MECs) for microbial production of hydrogen and value-added chemicals. Renew. Sust. Energ. Rev. 61, 501–525 (2016).
Sabatier, P. & Mailhe, A. Catayltic decompostion of formic acid. Compt. Rend. 152, 1212–1215 (1912).
Grasemann, M. & Laurenczy, G. Formic acid as a hydrogen source–recent developments and future trends. Energy Environ. Sci. 5, 8171–8181 (2012).
Zhu, Q.-L. & Xu, Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energy Environ. Sci. 8, 478–512 (2015).
Mellmann, D., Sponholz, P., Junge, H. & Beller, M. Formic acid as a hydrogen storage material—development of homogeneous catalysts for selective hydrogen release. Chem. Soc. Rev. 45, 3954–3988 (2016).
Taccardi, N. et al. Catalytic production of hydrogen from glucose and other carbohydrates under exceptionally mild reaction conditions. Green. Chem. 12, 1150–1156 (2010).
Li, Y., Sponholz, P., Nielsen, M., Junge, H. & Beller, M. Iridium-catalyzed hydrogen production from monosaccharides, disaccharide, cellulose, and lignocellulose. ChemSusChem 8, 804–808 (2015).
McGinnis, G. D., Prince, S. E., Biermann, C. J. & Lowrimore, J. T. Wet oxidation of model carbohydrate compounds. Carbohydr. Res. 128, 51–60 (1984).
Wölfel, R., Taccardi, N., Bösmann, A. & Wasserscheid, P. Selective catalytic conversion of biobased carbohydrates to formic acid using molecular oxygen. Green. Chem. 13, 2759–2763 (2011).
Jin, F. & Enomoto, H. Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions: chemistry of acid/base-catalysed and oxidation reactions. Energy Environ. Sci. 4, 382–397 (2011).
Li, J., Ding, D.-J., Deng, L., Guo, Q.-X. & Fu, Y. Catalytic air oxidation of biomass-derived carbohydrates to formic acid. ChemSusChem 5, 1313–1318 (2012).
Albert, J., Wölfel, R., Bösmann, A. & Wasserscheid, P. Selective oxidation of complex, water-insoluble biomass to formic acid using additives as reaction accelerators. Energy Environ. Sci. 5, 7956–7962 (2012).
Tang, Z. et al. Transformation of cellulose and its derived carbohydrates into formic and lactic acids catalyzed by vanadyl cations. ChemSusChem 7, 1557–1567 (2014).
Albert, J., Lüders, D., Bösmann, A., Guldi, D. M. & Wasserscheid, P. Spectroscopic and electrochemical characterization of heteropoly acids for their optimized application in selective biomass oxidation to formic acid. Green. Chem. 16, 226–237 (2014).
Wang, W. et al. Catalytic conversion of biomass-derived carbohydrates to formic acid using molecular oxygen. Green. Chem. 16, 2614–2618 (2014).
Niu, M., Hou, Y., Ren, S., Wu, W. & Marsh, K. N. Conversion of wheat straw into formic acid in NaVO3–H2SO4 aqueous solution with molecular oxygen. Green. Chem. 17, 453–459 (2015).
Reichert, J., Brunner, B., Jess, A., Wasserscheid, P. & Albert, J. Biomass oxidation to formic acid in aqueous media using polyoxometalate catalysts-boosting FA selectivity by in-situ extraction. Energy Environ. Sci. 8, 2985–2990 (2015).
Boddien, A. et al. Efficient dehydrogenation of formic acid using an iron catalyst. Science 333, 1733–1736 (2011).
Nielsen, M. et al. Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide. Nature 495, 85–89 (2013).
Wang, W.-H. et al. Formic acid dehydrogenation with bioinspired iridium complexes: a kinetic isotope effect study and mechanistic insight. ChemSusChem 7, 1976–1983 (2014).
Wang, W.-H. et al. Highly robust hydrogen generation by bioinspired Ir complexes for dehydrogenation of formic acid in water: experimental and theoretical mechanistic investigations at different pH. ACS Catal. 5, 5496–5504 (2015).
Wang, Z., Lu, S.-M., Li, J., Wang, J. & Li, C. Unprecedentedly high formic acid dehydrogenation activity on an iridium complex with an N,N′-diimine ligand in water. Chem. Eur. J. 21, 12592–12595 (2015).
Hull, J. F. et al. Reversible hydrogen storage using CO2 and a proton-switchable iridium catalyst in aqueous media under mild temperatures and pressures. Nat. Chem. 4, 383–388 (2012).
Maenaka, Y., Suenobu, T. & Fukuzumi, S. Catalytic interconversion between hydrogen and formic acid at ambient temperature and pressure. Energy Environ. Sci. 5, 7360–7367 (2012).
Boddien, A., Loges, B., Junge, H. & Beller, M. Hydrogen generation at ambient conditions: application in fuel cells. ChemSusChem 1, 751–758 (2008).
Harris, D. C. (ed.) Quantitative Chemical Analysis Ch. 7, 142–161 (W. H. Freeman and Company, New York, 2010).
Acknowledgements
This work was supported by National Nature Science Foundation of China (nos. 21472145 and 21305117) and a Leibniz fellowship. We thank W.-F. Tian, K.-H. He, Xi’an Jiaotong University for their help during the experiments. We thank L. Wang, Institute of Pulp and Paper Technology, Hubei University of Technology, China for affording various raw biomass and daily waste. We thank H.-J. Jiao, Leibniz-Institut für Katalyse e.V. an der Universität Rostock, Z.-J. Shi, Fudan University for helpful discussion. We thank Wattecs Lab Equipment Co., Ltd. for strong support on autoclaves and constant pressure gas collectors.
Author information
Authors and Affiliations
Contributions
Y.L. and M.B. conceived this project. P.Z., Y.-J.G., Y.-R.Z. and J.C. performed the experiments and analysed the data. J.C. synthesized the precursors of catalysts 5 and 6. P.Z., Y.-J.G., H.J., M.B. and Y.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisherʼs note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods; Supplementary Tables 1–8; Supplementary Figures 1–36; Supplementary references
Crystallographic data
Crystallographic data for compound [Cp*CF3IrCl2]2, CCDC reference 1522237
Rights and permissions
About this article
Cite this article
Zhang, P., Guo, YJ., Chen, J. et al. Streamlined hydrogen production from biomass. Nat Catal 1, 332–338 (2018). https://doi.org/10.1038/s41929-018-0062-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-018-0062-0
This article is cited by
-
Scalable electrosynthesis of commodity chemicals from biomass by suppressing non-Faradaic transformations
Nature Communications (2023)
-
Selective preparation for biofuels and high value chemicals based on biochar catalysts
Frontiers in Energy (2023)
-
Analysis of the hydrogen yield for the ethanol steam reforming
Biomass Conversion and Biorefinery (2023)
-
Catalytic conversion of glucose and its biopolymers into renewable compounds by inducing C–C bond scission and formation
Biomass Conversion and Biorefinery (2022)
-
The role of turmeric and bicnat on hydrogen production in porous tofu waste suspension electrolysis
Biomass Conversion and Biorefinery (2022)