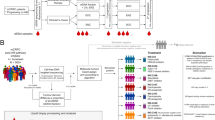
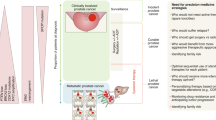
Abstract
Metastatic biopsy programmes combined with advances in genomic sequencing have provided new insights into the molecular landscape of castration-resistant prostate cancer (CRPC), identifying actionable targets, and emerging resistance mechanisms. The detection of DNA repair aberrations, such as mutation of BRCA2, could help select patients for poly(ADP-ribose) polymerase (PARP) inhibitor or platinum chemotherapy, and mismatch repair gene defects and microsatellite instability have been associated with responses to checkpoint inhibitor immunotherapy. Poor prognostic features, such as the presence of RB1 deletion, might help guide future therapeutic strategies. Our understanding of the molecular features of CRPC is now being translated into the clinic in the form of increased molecular testing for use of these agents and for clinical trial eligibility. Genomic testing offers opportunities for improving patient selection for systemic therapies and, ultimately, patient outcomes. However, challenges for precision oncology in advanced prostate cancer still remain, including the contribution of tumour heterogeneity, the timing and potential cooperation of multiple driver gene aberrations, and diverse resistant mechanisms. Defining the optimal use of molecular biomarkers in the clinic, including tissue-based and liquid biopsies, is a rapidly evolving field.
Key points
-
Studies investigating the genomic landscape of metastatic prostate cancer have identified targetable molecular alterations and emerging resistance mechanisms.
-
Alterations in the androgen receptor (AR) gene are a key driver of castration resistance in prostate cancer; AR mutation, amplification and the V7 splice variant can be detected non-invasively in patients, and have been associated with resistance to AR pathway inhibitors.
-
A subset of advanced prostate cancers harbour germline or somatic alterations involving DNA repair genes; homologous repair gene DNA repair defects have been associated with platinum chemotherapy and poly(ADP-ribose) polymerase (PARP) inhibitor sensitivity. Mismatch repair gene and CDK12 loss have been associated with responses to immunotherapy.
-
Combined loss of tumour suppressors RB1 and TP53 has been associated with lineage plasticity and the development of non-AR driven therapy resistance, which is enriched in tumours with small-cell and/or neuroendocrine pathological features on metastatic biopsy and aggressive clinical features.
-
Several biomarker-driven clinical trials are underway in patients with advanced prostate cancer that might ultimately lead to increasingly precise therapeutic strategies in patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019).
Lonergan, P. E. & Tindall, D. J. Androgen receptor signaling in prostate cancer development and progression. J. Carcinog. 10, 20 (2011).
Huggins, C. & Hodges, C. V. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 22, 232–240 (1972).
Sweeney, C. J. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N. Engl. J. Med. 373, 737–746 (2015).
James, N. D. et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N. Engl. J. Med. 377, 338–351 (2017).
Fizazi, K. et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 377, 352–360 (2017).
James, N. D. et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387, 1163–1177 (2016).
Watson, P. A., Arora, V. K. & Sawyers, C. L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 15, 701–711 (2015).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012).
Smith, M. R. et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl. J. Med. 378, 1408–1418 (2018).
Hussain, M. et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 378, 2465–2474 (2018).
Fizazi, K. et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 380, 1235–1246 (2019).
Loriot, Y. et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann. Oncol. 24, 1807–1812 (2013).
Noonan, K. L. et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann. Oncol. 24, 1802–1807 (2013).
Bluemn, E. G. et al. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell 32, 474–489.e6 (2017).
Aggarwal, R. et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J. Clin. Oncol. 36, 2492–2503 (2018).
Carreira, S. et al. Tumor clone dynamics in lethal prostate cancer. Sci. Transl Med. 6, 254ra125 (2014).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015).
Abida, W. et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl Acad. Sci. USA 116, 11428–11436 (2019).
Armenia, J. et al. The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 50, 645–651 (2018).
Beltran, H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat. Med. 22, 298–305 (2016).
Viswanathan, S. R. et al. Structural alterations driving castration-resistant prostate cancer revealed by linked-read genome sequencing. Cell 174, 433–447.e19 (2018).
Takeda, D. Y. et al. A somatically acquired enhancer of the androgen receptor is a noncoding driver in advanced prostate cancer. Cell 174, 422–432.e13 (2018).
Quigley, D. A. et al. Genomic hallmarks and structural variation in metastatic prostate Cancer. Cell 174, 758–769.e9 (2018).
Bohl, C. E., Gao, W., Miller, D. D., Bell, C. E. & Dalton, J. T. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc. Natl Acad. Sci. USA 102, 6201–6206 (2005).
Korpal, M. et al. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov. 3, 1030–1043 (2013).
Lallous, N. et al. Functional analysis of androgen receptor mutations that confer anti-androgen resistance identified in circulating cell-free DNA from prostate cancer patients. Genome Biol. 17, 10 (2016).
Romanel, A. et al. Plasma AR and abiraterone-resistant prostate cancer. Sci. Transl Med. 7, 312re310 (2015).
Azad, A. A. et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin. Cancer Res. 21, 2315–2324 (2015).
Conteduca, V. et al. Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration-resistant prostate cancer: a multi-institution correlative biomarker study. Ann. Oncol. 28, 1508–1516 (2017).
Annala, M. et al. Circulating Tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 8, 444–457 (2018).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Armstrong, A. J. et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J. Clin. Oncol. 37, 1120–1129 (2019).
Sharp, A. et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J. Clin. Invest. 129, 192–208 (2019).
Chang, K. H. et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell 154, 1074–1084 (2013).
Hearn, J. W. D. et al. HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol. 17, 1435–1444 (2016).
Hettel, D. & Sharifi, N. HSD3B1 status as a biomarker of androgen deprivation resistance and implications for prostate cancer. Nat. Rev. Urol. 15, 191–196 (2018).
Azad, A. A., Eigl, B. J., Murray, R. N., Kollmannsberger, C. & Chi, K. N. Efficacy of enzalutamide following abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer patients. Eur. Urol. 67, 23–29 (2015).
Attard, G. et al. Abiraterone alone or in combination with enzalutamide in metastatic castration-resistant prostate cancer with rising prostate-specific antigen during enzalutamide treatment. J. Clin. Oncol. 36, 2639–2646 (2018).
Morris, M. J. et al. Alliance A031201: a phase III trial of enzalutamide (ENZ) versus enzalutamide, abiraterone, and prednisone (ENZ/AAP) for metastatic castration resistant prostate cancer (mCRPC) [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 5008 (2019).
Andersen, R. J. et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell 17, 535–546 (2010).
Dalal, K. et al. Selectively targeting the DNA-binding domain of the androgen receptor as a prospective therapy for prostate cancer. J. Biol. Chem. 289, 26417–26429 (2014).
Zoubeidi, A. et al. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 67, 10455–10465 (2007).
Teply, B. A. et al. Bipolar androgen therapy in men with metastatic castration-resistant prostate cancer after progression on enzalutamide: an open-label, phase 2, multicohort study. Lancet Oncol. 19, 76–86 (2018).
Chatterjee, P. et al. Supraphysiological androgens suppress prostate cancer growth through androgen receptor-mediated DNA damage. J. Clin. Invest. https://doi.org/10.1172/JCI127613 (2019).
Boysen, G. et al. SPOP-mutated/CHD1-deleted lethal prostate cancer and abiraterone sensitivity. Clin. Cancer Res. 24, 5585–5593 (2018).
Liu, D. et al. Impact of the SPOP mutant subtype on the interpretation of clinical parameters in prostate cancer. JCO Precis. Oncol. 2, 1–13 (2018).
Blattner, M. et al. SPOP mutation drives prostate tumorigenesis in vivo through coordinate regulation of PI3K/mTOR and AR signaling. Cancer Cell 31, 436–451 (2017).
Carpten, J. D. et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature 448, 439–444 (2007).
Hyman, D. M. et al. AKT inhibition in solid tumors with AKT1 mutations. J. Clin. Oncol. 35, 2251–2259 (2017).
Ferraldeschi, R. et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur. Urol. 67, 795–802 (2015).
Carver, B. S. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 (2011).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03072238 (2019).
Pritchard, C. C. et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N. Engl. J. Med. 375, 443–453 (2016).
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines®). Prostate cancer version 4.2019. NCCN https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (2019).
Cheng, H. H., Sokolova, A. O., Schaeffer, E. M., Small, E. J. & Higano, C. S. Germline and somatic mutations in prostate cancer for the clinician. J. Natl. Compr. Canc. Netw. 17, 515–521 (2019).
Lord, C. J. & Ashworth, A. PARP inhibitors: synthetic lethality in the clinic. Science 355, 1152–1158 (2017).
Konstantinopoulos, P. A., Ceccaldi, R., Shapiro, G. I. & D’Andrea, A. D. Homologous recombination deficiency: exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 5, 1137–1154 (2015).
Mateo, J. et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 373, 1697–1708 (2015).
Kumar, A. et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 22, 369–378 (2016).
Cheng, H. H., Pritchard, C. C., Boyd, T., Nelson, P. S. & Montgomery, B. Biallelic inactivation of BRCA2 in platinum-sensitive metastatic castration-resistant prostate cancer. Eur. Urol. 69, 992–995 (2016).
Pomerantz, M. M. et al. The association between germline BRCA2 variants and sensitivity to platinum-based chemotherapy among men with metastatic prostate cancer. Cancer 123, 3532–3539 (2017).
Zafeiriou, Z. et al. Genomic analysis of three metastatic prostate cancer patients with exceptional responses to carboplatin indicating different types of DNA repair deficiency. Eur. Urol. 75, 184–192 (2019).
D’Andrea, A. D. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair 71, 172–176 (2018).
Goodall, J. et al. Circulating cell-free DNA to guide prostate cancer treatment with PARP inhibition. Cancer Discov. 7, 1006–1017 (2017).
Quigley, D. et al. Analysis of circulating cell-free DNA identifies multiclonal heterogeneity of BRCA2 reversion mutations associated with resistance to PARP inhibitors. Cancer Discov. 7, 999–1005 (2017).
Abida, W. et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 5, 471–478 (2019).
Nava Rodrigues, D. et al. Immunogenomic analyses associate immunological alterations with mismatch repair defects in prostate cancer. J. Clin. Invest. 128, 4441–4453 (2018).
Boyiadzis, M. M. et al. Significance and implications of FDA approval of pembrolizumab for biomarker-defined disease. J. Immunother Cancer 6, 35 (2018).
Wu, Y. M. et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell 173, 1770–1782.e14 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03570619 (2019).
Malumbres, M. & Barbacid, M. Cell cycle, CDKs and cancer: a changing paradigm. Nat. Rev. Cancer 9, 153–166 (2009).
Comstock, C. E. et al. Targeting cell cycle and hormone receptor pathways in cancer. Oncogene 32, 5481–5491 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02905318 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02555189 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02059213 (2019).
Chinnam, M. & Goodrich, D. W. RB1, development, and cancer. Curr. Top. Dev. Biol. 94, 129–169 (2011).
Aparicio, A. M. et al. Combined tumor suppressor defects characterize clinically defined aggressive variant prostate cancers. Clin. Cancer Res. 22, 1520–1530 (2016).
Ku, S. Y. et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78–83 (2017).
Mu, P. et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 355, 84–88 (2017).
Beltran, H. et al. The role of lineage plasticity in prostate cancer therapy resistance. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-19-1423 (2019).
Palmgren, J. S., Karavadia, S. S. & Wakefield, M. R. Unusual and underappreciated: small cell carcinoma of the prostate. Semin. Oncol. 34, 22–29 (2007).
Epstein, J. I. et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am. J. Surg. Pathol. 38, 756–767 (2014).
Beltran, H. et al. A phase 2 study of the aurora kinase A inhibitor alisertib for patients with neuroendocrine prostate cancer (NEPC) [abstract]. Ann. Oncol. 27 (Suppl. 6), LBA29 (2016).
Gong, X. et al. Aurora A kinase inhibition is synthetic lethal with loss of the RB1 tumor suppressor gene. Cancer Discov. 9, 248–263 (2019).
Puca, L. et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci. Transl Med. 11, eaav0891 (2019).
Baca, S. C. et al. Punctuated evolution of prostate cancer genomes. Cell 153, 666–677 (2013).
Taylor, R. A. et al. Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories. Nat. Commun. 8, 13671 (2017).
Nava Rodrigues, D. et al. RB1 heterogeneity in advanced metastatic castration-resistant prostate cancer. Clin. Cancer Res. 25, 687–697 (2019).
Easwaran, H., Tsai, H. C. & Baylin, S. B. Cancer epigenetics: tumor heterogeneity, plasticity of stem-like states, and drug resistance. Mol. Cell 54, 716–727 (2014).
Varambally, S. et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 419, 624–629 (2002).
Beltran, H. et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 1, 487–495 (2011).
Dardenne, E. et al. N-myc Induces an EZH2-mediated transcriptional program driving neuroendocrine prostate cancer. Cancer Cell 30, 563–577 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03480646 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03460977 (2019).
Stathis, A. & Bertoni, F. BET proteins as targets for anticancer treatment. Cancer Discov. 8, 24–36 (2018).
Asangani, I. A. et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature 510, 278–282 (2014).
Welti, J. et al. Targeting bromodomain and extra-terminal (BET) family proteins in castration-resistant prostate cancer (CRPC). Clin. Cancer. Res. 24, 3149–3162 (2018).
Asangani, I. A. et al. BET bromodomain inhibitors enhance efficacy and disrupt resistance to AR antagonists in the treatment of prostate cancer. Mol. Cancer. Res. 14, 324–331 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02711956 (2019).
Shi, Y. et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953 (2004).
Metzger, E. et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437, 436–439 (2005).
Sehrawat, A. et al. LSD1 activates a lethal prostate cancer gene network independently of its demethylase function. Proc. Natl Acad. Sci. USA 115, E4179–E4188 (2018).
Cai, C. et al. Lysine-specific demethylase 1 has dual functions as a major regulator of androgen receptor transcriptional activity. Cell Rep. 9, 1618–1627 (2014).
Haffner, M. C. et al. Tracking the clonal origin of lethal prostate cancer. J. Clin. Invest. 123, 4918–4922 (2013).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
Aryee, M. J. et al. DNA methylation alterations exhibit intraindividual stability and interindividual heterogeneity in prostate cancer metastases. Sci. Transl Med. 5, 169ra110 (2013).
Wyatt, A. W. et al. Genomic alterations in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. JAMA Oncol. 2, 1598–1606 (2016).
Salvi, S. et al. Circulating AR copy number and outcome to enzalutamide in docetaxel-treated metastatic castration-resistant prostate cancer. Oncotarget 7, 37839–37845 (2016).
Conteduca, V. et al. Plasma androgen receptor (pAR) status and activity of taxanes in metastatic castration resistant prostate cancer (mCRPC) [abstract]. J. Clin. Oncol. 36 (15 Suppl.), 5074–5074 (2018).
Conteduca, V. et al. Plasma androgen receptor and serum chromogranin A in advanced prostate cancer. Sci. Rep. 8, 15442 (2018).
Wyatt, A. W. et al. Concordance of circulating tumor DNA and matched metastatic tissue biopsy in prostate cancer. J. Natl Cancer Inst. 109, djx118 (2017).
Beltran, H. et al. The initial detection and partial characterization of circulating tumor cells in neuroendocrine prostate cancer. Clin. Cancer Res. 22, 1510–1519 (2016).
Lambros, M. B. et al. Single-cell analyses of prostate cancer liquid biopsies acquired by apheresis. Clin. Cancer Res. 24, 5635–5644 (2018).
Acknowledgements
The authors acknowledge research support from the Prostate Cancer Foundation (S.-Y.K., M.E.G., H.B.), the Terry Fox Research Institute (M.E.G.), Prostate Cancer Canada (M.E.G.), the National Cancer Institute SPORE (H.B.) and the Department of Defense Prostate Cancer Research Program (H.B.).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussion of content, and wrote, reviewed, and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
H.B. has received research funding from Janssen, Abbvie Stemcentryx, Astellas, Eli Lilly and Millennium, and has served as advisor/consultant for Janssen, Astellas, Amgen, Astra Zeneca and Sanofi Genzyme. M.E.G is listed as inventor on patents granted to the University of British Columbia on antisense and small-molecule inhibitors of HSP27 for the treatment of cancer. S.-Y. K. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ku, SY., Gleave, M.E. & Beltran, H. Towards precision oncology in advanced prostate cancer. Nat Rev Urol 16, 645–654 (2019). https://doi.org/10.1038/s41585-019-0237-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-019-0237-8
This article is cited by
-
Development and validation of a survival nomogram and calculator for male patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate and/or enzalutamide
BMC Cancer (2023)
-
Identification of the mitochondrial protein POLRMT as a potential therapeutic target of prostate cancer
Cell Death & Disease (2023)
-
CHRM4/AKT/MYCN upregulates interferon alpha-17 in the tumor microenvironment to promote neuroendocrine differentiation of prostate cancer
Cell Death & Disease (2023)
-
Novel device for dividing core needle biopsy specimens to provide paired mirror image-like tissues for genetic and pathological tests
Scientific Reports (2023)
-
A potassium-chloride co-transporter promotes tumor progression and castration resistance of prostate cancer through m6A reader YTHDC1
Cell Death & Disease (2023)