Abstract
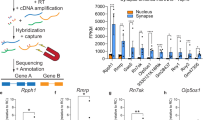
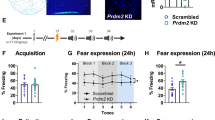
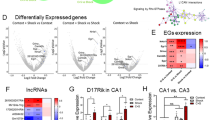
DNA forms conformational states beyond the right-handed double helix; however, the functional relevance of these noncanonical structures in the brain remains unknown. Here we show that, in the prefrontal cortex of mice, the formation of one such structure, Z-DNA, is involved in the regulation of extinction memory. Z-DNA is formed during fear learning and reduced during extinction learning, which is mediated, in part, by a direct interaction between Z-DNA and the RNA-editing enzyme Adar1. Adar1 binds to Z-DNA during fear extinction learning, which leads to a reduction in Z-DNA at sites where Adar1 is recruited. Knockdown of Adar1 leads to an inability to modify a previously acquired fear memory and blocks activity-dependent changes in DNA structure and RNA state—effects that are fully rescued by the introduction of full-length Adar1. These findings suggest a new mechanism of learning-induced gene regulation that is dependent on proteins that recognize alternate DNA structure states, which are required for memory flexibility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data and code used to generate the data are available upon reasonable request to the authors.
Change history
26 June 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41593-020-0669-8
References
Schwartz, T., Rould, M. A., Lowenhaupt, K., Herbert, A. & Rich, A. Crystal structure of the Zα domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science 284, 1841–1845 (1999).
Herbert, A. et al. The Zα domain from human ADAR1 binds to the Z-DNA conformer of many different sequences. Nucleic Acids Res. 26, 3486–3493 (1998).
Crick, F. H. C. & Watson, J. D. The complementary structure of deoxyribonucleic acid. Proc. R. Soc. A Math. Phys. Eng. Sci. 223, 80–96 (1954).
Franklin, R. E. & Gosling, R. G. The structure of sodium thymonucleate fibres. I. The influence of water content. Acta Crystallogr. 6, 673–677 (1953).
Wang, A. H. J. et al. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature 282, 680–686 (1979).
Pohl, F. M. Hysteretic behaviour of a Z-DNA-antibody complex. Biophys. Chem. 26, 385–390 (1987).
Herbert, A. A genetic instruction code based on DNA conformation. Trends Genet. 35, 887–890 (2019).
Haniford, D. B. & Pulleyblank, D. E. Facile transition of poly[d(TG)·d(CA)] into a left-handed helix in physiological conditions. Nature 302, 632–634 (1983).
Dobi, A. & Agoston, D. V. Submillimolar levels of calcium regulates DNA structure at the dinucleotide repeat (TG/AC)n. Chem. Biochem. 95, 5981–5986 (1998).
Behe, M. & Felsenfeld, G. Effects of methylation on a synthetic polynucleotide: the B–Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc. Natl Acad. Sci. USA 78, 1619–1623 (1981).
Marshall, P. & Bredy, T. W. Cognitive neuroepigenetics: the next evolution in our understanding of the molecular mechanisms underlying learning and memory? NPJ Sci. Learn. 1, 16014 (2016).
McGaugh, J. L. Memory–a century of consolidation. Science 287, 248–251 (2000).
Schade, M. et al. The solution structure of the Zα domain of the human RNA editing enzyme ADAR1 reveals a prepositioned binding surface for Z-DNA. Proc. Natl Acad. Sci. USA 96, 12465–12470 (1999).
Lee, Y. M. et al. NMR investigation on the DNA binding and B–Z transition pathway of the Zα domain of human ADAR1. Biophys. Chem. 172, 18–25 (2013).
Lee, A.-R. et al. NMR dynamics study reveals the Zα domain of human ADAR1 associates with and dissociates from Z-RNA more slowly than Z-DNA. ACS Chem. Biol. 14, 245–255 (2019).
Rudenko, A. et al. Tet1 is critical for neuronal activity-regulated gene expression and memory extinction. Neuron 79, 1109–1122 (2013).
Marshall, P. R. & Bredy, T. W. Neuroepigenetic mechanisms underlying fear extinction: emerging concepts. Psychopharmacology 236, 133–142 (2018).
Li, X. et al. The DNA modification N6-methyl-2′-deoxyadenosine (m6dA) drives activity-induced gene expression and is required for fear extinction. Nat. Neurosci. 22, 534–544 (2019).
Nie, Y., Zhao, Q., Su, Y. & Yang, J. H. Subcellular distribution of AdAR1 isoforms is synergistically determined by three nuclear discrimination signals and a regulatory motif. J. Biol. Chem. 279, 13249–13255 (2004).
Keum, S. et al. A missense variant at the Nrxn3 locus enhances empathy fear in the mouse. Neuron 98, 588–601 (2018).
Thomas, T. J., Gunnia, U. B. & Thomas, T. Polyamine-induced B-DNA to Z-DNA conformational transition of a plasmid DNA with (dG-dC)(n) insert. J. Biol. Chem. 266, 6137–6141 (1991).
Van Helden, P. D. The effect of adriamycin on Z-DNA formation and DNA synthesis. Nucleic Acids Res. 11, 8415–8420 (1983).
Dieci, G., Fiorino, G., Castelnuovo, M., Teichmann, M. & Pagano, A. The expanding RNA polymerase III transcriptome. Trends Genet. 23, 614–622 (2007).
Champ, P. C., Maurice, S., Vargason, J. M., Camp, T. & Ho, P. S. Distributions of Z-DNA and nuclear factor I in human chromosome 22: a model for coupled transcriptional regulation. Nucleic Acids Res. 32, 6501–6510 (2004).
Naylor, L. H. & Clark, E. M. D(TG)n·d(CA)n sequences upstream of the rat prolactin gene form z-DNA and inhibit gene transcription. Nucleic Acids Res. 18, 1595–1601 (1990).
Rich, A. & Zhang, S. Z-DNA: the long road to biological function. Nat. Rev. Genet. 4, 566–572 (2003).
Shin, S. I. et al. Z-DNA-forming sites identified by ChIP-Seq are associated with actively transcribed regions in the human genome. DNA Res. 23, 477–486 (2016).
Wang, G. & Vasquez, K. M. Non-B DNA structure-induced genetic instability. Mutat. Res. - Fundam. Mol. Mech. Mutagen. 598, 103–119 (2006).
Harteis, S. & Schneider, S. Making the bend: DNA tertiary structure and protein–DNA interactions. Int. J. Mol. Sci. 15, 12335–12363 (2014).
Rohs, R. et al. The role of DNA shape in protein-DNA recognition. Nature 461, 1248–1253 (2009).
Berber, I. et al. Spectroscopic characterization of a DNA-binding domain, Zα, from the editing enzyme, dsRNA adenosine deaminase: evidence for left-handed Z-DNA in the Zα-DNA complex. Biochemistry 37, 13313–13321 (1998).
Bae, S., Kim, D., Kim, K. K., Kim, Y. G. & Hohng, S. Intrinsic Z-DNA is stabilized by the conformational selection mechanism of Z-DNA-binding proteins. J. Am. Chem. Soc. 133, 668–671 (2011).
Bae, S. et al. Energetics of Z-DNA binding protein-mediated helicity reversals in DNA, RNA, and DNA–RNA duplexes. J. Phys. Chem. B 117, 13866–13871 (2013).
Antrup, H. & Seiler, N. On the turnover of polyamines spermidine and spermine in mouse brain and other organs. Neurochem. Res. 5, 123–143 (1980).
Paleček, E. Local supercoil-stabilized DNA structure. Crit. Rev. Biochem. Mol. Biol. 26, 151–226 (1991).
Rahmouni, A. R. & Wells, R. D. Stabilization of Z DNA in vivo by localized supercoiling. Science 246, 358–363 (1989).
Madabhushi, R. et al. Activity-induced DNA breaks govern the expression of neuronal early-response genes. Cell 161, 1592–1605 (2015).
Li, X. et al. The DNA repair-associated protein Gadd45ɣ regulates the temporal coding of immediate early gene expression within the prelimbic prefrontal cortex and is required for the consolidation of associative fear memory. J. Neurosci. 39, 970–983 (2018).
Wang, G., Christensen, L. A. & Vasquez, K. M. Z-DNA-forming sequences generate large-scale deletions in mammalian cells. PNAS 103, 2677–2682 (2006).
Ditlevson, J. V. et al. Inhibitory effect of a short Z-DNA forming sequence on transcription elongation by T7 RNA polymerase. Nucleic Acids Res. 36, 3163–3170 (2008).
Prigogine, I. Time, structure, and fluctuations. Science 201, 777–785 (1978).
Lu, J. Y. et al. L1 and B1 repeats blueprint the spatial organization of chromatin. Preprint at bioRxiv https://doi.org/10.1101/802173 (2019).
Oh, D.-B., Kim, Y.-G. & Rich, A. Z-DNA-binding proteins can act as potent effectors of gene expression in vivo. Proc. Natl Acad. Sci. USA 99, 16666–16671 (2002).
Rothenburg, S. et al. A PKR-like eukaryotic initiation factor 2 kinase from zebrafish contains Z-DNA binding domains instead of dsRNA binding domains. Proc. Natl Acad. Sci. USA 102, 1602–1607 (2005).
Wölfl, S., Martinez, C., Rich, A. & Majzoub, J. A. Transcription of the human corticotropin-releasing hormone gene in NPLC cells is correlated with Z-DNA formation. Proc. Natl Acad. Sci. USA 93, 3664–3668 (1996).
Kang, H. J. et al. Novel interaction of the Z-DNA binding domain of human ADAR1 with the oncogenic c-myc promoter G-quadruplex. J. Mol. Biol. 426, 2594–2604 (2014).
Liddicoat, B. J. et al. RNA editing by ADAR1 prevents MDA5 sensing of endogenous dsRNA as nonself. Science 349, 1115–1120 (2015).
Lin, Q. et al. The brain-specific microRNA miR-128b regulates the formation of fear-extinction memory. Nat. Neurosci. 14, 1115–1117 (2011).
O’Neill, L. P., VerMilyea, M. D. & Turner, B. M. Epigenetic characterization of the early embryo with a chromatin immunoprecipitation protocol applicable to small cell populations. Nat. Genet. 38, 835–841 (2006).
Acknowledgements
The authors gratefully acknowledge grant support from the National Institutes of Health (R01MH105398-TWB) and the National Health and Medical Research Council (GNT1145172 and GNT1160823-TWB), the ARC (GNT190101078-XL), the Westpac Future Scholars program (E.L.Z., L.J.L. and S.U.M.) and postgraduate scholarships from the Natural Sciences and Engineering Research Council (P.R.M.) and the University of Queensland (P.R.M., E.L.Z., L.J.L., D.B., J.Y. and S.U.M.). We would also like to thank R. Tweedale for helpful editing of the manuscript.
Author information
Authors and Affiliations
Contributions
P.R.M designed and performed all wet lab and behavioral experiments and wrote the manuscript with T.W.B. Q.Z. performed all the bioinformatics analysis. X.L. and W.W. assisted in experimental design and ChIP-seq experiments. A.P. assisted in the production of lentivirus. E.Z, L.J.L. and S.U.M. assisted with behavioral experiments. D.B. assisted with bioinformatic analysis. Z.W., J.Y. and W.S.L. assisted with western blots and qPCR. A.G. assisted in producing and validating the ADAR1 mutant constructs. C.W. contributed reagents and helped write the manuscript. T.W.B. conceived the study, designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Neuroscience thanks Alan Herbert, Larry Zweifel and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Data from this paper can be accessed at PRJNA545193.
Supplementary information
Supplemental Information
Supplementary Figs. 1–9 and Supplementary Tables 1–3.
.Supplementary Table
Supplementary Table 1. Genome-wide ADAR1 significant peaks in Excel file.
Source data
Source Data Fig. 1
Unprocessed western blots for Fig. 1
Rights and permissions
About this article
Cite this article
Marshall, P.R., Zhao, Q., Li, X. et al. Dynamic regulation of Z-DNA in the mouse prefrontal cortex by the RNA-editing enzyme Adar1 is required for fear extinction. Nat Neurosci 23, 718–729 (2020). https://doi.org/10.1038/s41593-020-0627-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-020-0627-5
This article is cited by
-
AIRE relies on Z-DNA to flag gene targets for thymic T cell tolerization
Nature (2024)
-
Dynamic alternative DNA structures in biology and disease
Nature Reviews Genetics (2023)
-
Fear extinction is regulated by the activity of long noncoding RNAs at the synapse
Nature Communications (2023)
-
New Insights into the Functions of Nucleic Acids Controlled by Cellular Microenvironments
Topics in Current Chemistry (2021)
-
A-to-Z interactions in fear extinction
Nature Reviews Neuroscience (2020)