Abstract
The global decline in malaria has stalled1, emphasizing the need for vaccines that induce durable sterilizing immunity. Here we optimized regimens for chemoprophylaxis vaccination (CVac), for which aseptic, purified, cryopreserved, infectious Plasmodium falciparum sporozoites (PfSPZ) were inoculated under prophylactic cover with pyrimethamine (PYR) (Sanaria PfSPZ-CVac(PYR)) or chloroquine (CQ) (PfSPZ-CVac(CQ))—which kill liver-stage and blood-stage parasites, respectively—and we assessed vaccine efficacy against homologous (that is, the same strain as the vaccine) and heterologous (a different strain) controlled human malaria infection (CHMI) three months after immunization (https://clinicaltrials.gov/, NCT02511054 and NCT03083847). We report that a fourfold increase in the dose of PfSPZ-CVac(PYR) from 5.12 × 104 to 2 × 105 PfSPZs transformed a minimal vaccine efficacy (low dose, two out of nine (22.2%) participants protected against homologous CHMI), to a high-level vaccine efficacy with seven out of eight (87.5%) individuals protected against homologous and seven out of nine (77.8%) protected against heterologous CHMI. Increased protection was associated with Vδ2 γδ T cell and antibody responses. At the higher dose, PfSPZ-CVac(CQ) protected six out of six (100%) participants against heterologous CHMI three months after immunization. All homologous (four out of four) and heterologous (eight out of eight) infectivity control participants showed parasitaemia. PfSPZ-CVac(CQ) and PfSPZ-CVac(PYR) induced a durable, sterile vaccine efficacy against a heterologous South American strain of P. falciparum, which has a genome and predicted CD8 T cell immunome that differs more strongly from the African vaccine strain than other analysed African P. falciparum strains.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information and from the corresponding author upon reasonable request.
References
WHO. World Malaria Report 2019 (World Health Organization, 2019).
The RTS,S Clinical Trials Partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med. 11, e1001685 (2014).
RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 386, 31–45 (2015).
The RTS,S Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 367, 2284–2295 (2012).
Epstein, J. E. et al. Protection against Plasmodium falciparum malaria by PfSPZ vaccine. JCI Insight 2, e89154 (2017).
Jongo, S. A. et al. Safety, immunogenicity, and protective efficacy against controlled human malaria infection of Plasmodium falciparum sporozoite vaccine in Tanzanian adults. Am. J. Trop. Med. Hyg. 99, 338–349 (2018).
Lyke, K. E. et al. Attenuated PfSPZ vaccine induces strain-transcending T cells and durable protection against heterologous controlled human malaria infection. Proc. Natl Acad. Sci. USA 114, 2711–2716 (2017).
Seder, R. A. et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science 341, 1359–1365 (2013).
Sissoko, M. S. et al. Safety and efficacy of PfSPZ vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect. Dis. 17, 498–509 (2017).
Sissoko, M. S. et al. Three dose regimen of PfSPZ vaccine protects adult Malians against Plasmodium falciparum through an intense transmission season: a randomised, controlled phase I trial. Lancet Infect. Dis. (in the press).
Butler, N. S. et al. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9, 451–462 (2011).
Kublin, J. G. et al. Complete attenuation of genetically engineered sporozoites in human subjects. Sci. Transl. Med. 9, eaad9099 (2017).
Roestenberg, M. et al. A double-blind, placebo-controlled phase 1/2a trial of the genetically attenuated malaria vaccine PfSPZ-GA1. Sci. Transl. Med. 12, eaaz5629 (2020).
Roestenberg, M. et al. Protection against a malaria challenge by sporozoite inoculation. N. Engl. J. Med. 361, 468–477 (2009).
Bijker, E. M. et al. Sporozoite immunization of human volunteers under mefloquine prophylaxis is safe, immunogenic and protective: a double-blind randomized controlled clinical trial. PLoS ONE 9, e112910 (2014).
Sahu, T. et al. Chloroquine neither eliminates liver stage parasites nor delays their development in a murine chemoprophylaxis vaccination model. Front. Microbiol. 6, 283 (2015).
Hoffman, S. L. et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 185, 1155–1164 (2002).
Mordmüller, B. et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 542, 445–449 (2017).
Schats, R. et al. Heterologous protection against malaria after immunization with Plasmodium falciparum sporozoites. PLoS ONE 10, e0124243 (2015).
Walk, J. et al. Modest heterologous protection after Plasmodium falciparum sporozoite immunization: a double-blind randomized controlled clinical trial. BMC Med. 15, 168 (2017).
Friesen, J., Borrmann, S. & Matuschewski, K. Induction of antimalaria immunity by pyrimethamine prophylaxis during exposure to sporozoites is curtailed by parasite resistance. Antimicrob. Agents Chemother. 55, 2760–2767 (2011).
Friesen, J. & Matuschewski, K. Comparative efficacy of pre-erythrocytic whole organism vaccine strategies against the malaria parasite. Vaccine 29, 7002–7008 (2011).
Healy, S. A. et al. Chemoprophylaxis vaccination: phase I study to explore stage-specific immunity to Plasmodium falciparum in US adults. Clin. Infect. Dis. 71, 1481–1490 (2020).
Epstein, J. E. & Richie, T. L. The whole parasite, pre-erythrocytic stage approach to malaria vaccine development: a review. Curr. Opin. Infect. Dis. 26, 420–428 (2013).
March, S. et al. A microscale human liver platform that supports the hepatic stages of Plasmodium falciparum and vivax. Cell Host Microbe 14, 104–115 (2013).
March, S. et al. Micropatterned coculture of primary human hepatocytes and supportive cells for the study of hepatotropic pathogens. Nat. Protocols 10, 2027–2053 (2015).
Gural, N. et al. In vitro culture, drug sensitivity, and transcriptome of Plasmodium vivax hypnozoites. Cell Host Microbe 23, 395–406.e4 (2018).
White, N. J. Clinical pharmacokinetics of antimalarial drugs. Clin. Pharmacokinet. 10, 187–215 (1985).
Ishizuka, A. S. et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat. Med. 22, 614–623 (2016).
Zaidi, I. et al. γδ T cells are required for the induction of sterile immunity during irradiated sporozoite vaccinations. J. Immunol. 199, 3781–3788 (2017).
Nahrendorf, W., Scholzen, A., Sauerwein, R. W. & Langhorne, J. Cross-stage immunity for malaria vaccine development. Vaccine 33, 7513–7517 (2015).
Nahrendorf, W. et al. Blood-stage immunity to Plasmodium chabaudi malaria following chemoprophylaxis and sporozoite immunization. eLife 4, e05165 (2015).
Moser, K. A. et al. Strains used in whole organism Plasmodium falciparum vaccine trials differ in genome structure, sequence, and immunogenic potential. Genome Med. 12, 6 (2020).
Bijker, E. M. et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc. Natl Acad. Sci. USA 110, 7862–7867 (2013).
Belnoue, E. et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 172, 2487–2495 (2004).
Lyke, K. E. et al. Multidose priming and delayed boosting improve Plasmodium falciparum sporozoite vaccine efficacy against heterologous P. falciparum controlled human malaria infection. Clin. Infect. Dis. ciaa1294 (2020).
Hoffman, S. L. & Doolan, D. L. Malaria vaccines-targeting infected hepatocytes. Nat. Med. 6, 1218–1219 (2000).
Epstein, J. E. et al. Live attenuated malaria vaccine designed to protect through hepatic CD8+ T cell immunity. Science 334, 475–480 (2011).
Issiaka, D. et al. Impact of seasonal malaria chemoprevention on hospital admissions and mortality in children under 5 years of age in Ouelessebougou, Mali. Malar. J. 19, 103 (2020).
Jongo, S. A. et al. Safety and differential antibody and T-cell responses to the Plasmodium falciparum sporozoite malaria vaccine, PfSPZ vaccine, by age in Tanzanian adults, adolescents, children, and infants. Am. J. Trop. Med. Hyg. 100, 1433–1444 (2019).
Sulyok, Z. et al. Heterologous protection against malaria by a simple chemoattenuated PfSPZ vaccine regimen in a randomized trial. Nat. Commun. 12, 2518 (2021)
Spring, M. et al. First-in-human evaluation of genetically attenuated Plasmodium falciparum sporozoites administered by bite of Anopheles mosquitoes to adult volunteers. Vaccine 31, 4975–4983 (2013).
Goswami, D. et al. A replication-competent late liver stage-attenuated human malaria parasite. JCI Insight 5, e135589 (2020).
Roestenberg, M. et al. Controlled human malaria infections by intradermal injection of cryopreserved Plasmodium falciparum sporozoites. Am. J. Trop. Med. Hyg. 88, 5–13 (2013).
Mordmüller, B. et al. Direct venous inoculation of Plasmodium falciparum sporozoites for controlled human malaria infection: a dose-finding trial in two centres. Malaria J. 14, 117 (2015).
Laurens, M. B. et al. Dose-dependent infectivity of aseptic, purified, cryopreserved Plasmodium falciparum 7G8 sporozoites in malaria-naive adults. J. Infect. Dis. 220, 1962–1966 (2019).
Divis, P. C., Shokoples, S. E., Singh, B. & Yanow, S. K. A TaqMan real-time PCR assay for the detection and quantitation of Plasmodium knowlesi. Malar. J. 9, 344 (2010).
Seilie, A. M. et al. Beyond blood smears: qualification of Plasmodium 18S rRNA as a biomarker for controlled human malaria infections. Am. J. Trop. Med. Hyg. 100, 1466–1476 (2019).
Luethy, P. M. et al. Diagnostic challenges of prolonged post-treatment clearance of Plasmodium nucleic acids in a pre-transplant autosplenectomized patient with sickle cell disease. Malar. J. 17, 23 (2018).
Rougemont, M. et al. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J. Clin. Microbiol. 42, 5636–5643 (2004).
Gabriel, E. E., Nason, M., Fay, M. P. & Follmann, D. A. A boundary-optimized rejection region test for the two-sample binomial problem. Stat. Med. 37, 1047–1058 (2018).
Acknowledgements
We thank the participants of the vaccine trial for their contribution and commitment to vaccine research. This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health and by the NIAID grants U01AI109700-01 and 2R44AI058375-11 to Sanaria for vaccine manufacture and trial execution. S.N.B. is a Howard Hughes Medical Institute Investigator. We thank the Sanaria and Protein Potential legal, administrative, manufacturing, quality, logistics, pharmaceutical operations, regulatory and clinical teams for their support of this project; T. Bauch, U. Desai, M. Mahoney, L. Walker, L. Williams of the Department of Laboratory Medicine, NIH CC for performing the malaria PCR diagnostic tests; N. Nerurkar for establishing and infecting the micropatterned co-cultures of hepatocytes; the LMIV and NIH CC clinical, pharmacy, immunology, laboratory, data, sample and project management teams and, in particular, NIH CC Outpatient Clinic 8 and Day Hospital Staffing; and J. Patrick Gorres for coordinating the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
S.A.H., S.L.H. and P.E.D. conceived and designed the study. A.M.-O., S.A.H., J.L., D.M.C., S.K., C.W., A.K., O.M.-S., A.I., L.K.D., H.D. and J.E.J. executed the clinical trials, with safety oversight by L.W.C.P. and T.L.R. T.M., A.M., A.G., B.K.L.S., P.F.B. and E.R.J. prepared the investigational product and coordinated regulatory affairs. M.N. analysed the clinical trial results. J.D., J.N., J.R., G.L., J.C.C.H., N.K., S.C., I.I., C.A., S.C.M., S.M., S.N.B. and I.Z. designed and performed laboratory studies. A.M.-O., S.L.H. and P.E.D. prepared the initial manuscript draft; all authors reviewed and edited the manuscript. A.M.-O. and S.A.H. supervised the trials and S.L.H. and P.E.D. supervised the study teams.
Corresponding author
Ethics declarations
Competing interests
T.M., L.W.P.C., A.M., A.G., B.K.L.S., N.K.C., S.C., P.F.B., E.R.J., T.L.R. and S.L.H. are salaried, full-time employees of Sanaria, the developer and sponsor of Sanaria PfSPZ Vaccine. S.L.H. and B.K.L.S. also have financial interests in Sanaria. B.K.L.S. and S.L.H. are inventors on patents and patent applications that have been assigned to Sanaria. All other authors declare no competing interests.
Additional information
Peer review information Nature thanks Stefan Kappe and Laurent Renia for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Trial profile for PfSPZ-CVac low-dose study.
αOne participant was treated at day 16 after CHMI although that participant did complete the end-of-study visit. βThe serious adverse event (SAE) and adverse event (AE) in the PYR/CQ group were encephalopathy and eye irritation, respectively. The non-compliance incidents include a participant’s unwillingness to remain on pregnancy prevention (PYR/CQ group) and a work conflict preventing the participant from attending scheduled visits (CQ group).
Extended Data Fig. 2 Detection of parasitaemia in volunteers by qPCR after low-dose PfSPZ-CVac.
Parasitaemia was measured in participants on days 6–10 after each PfSPZ-CVac dose. The median parasitaemia is displayed for positive participants and error bars indicate the IQR. Vax, PfSPZ Challenge + CQ or PYR/CQ administration and follow-up until day 14 after PfSPZ Challenge 1 and day 10 after PfSPZ Challenge doses 2 and 3. The table shows (from left to right in each cell): the number of participants who were positive by qPCR/the injected number of participants; the median peak parasite density of positive participants (parasites per ml); and the average day of peak parasite density after each dose of PfSPZ-CVac. ND, not detected; n/a, not applicable.
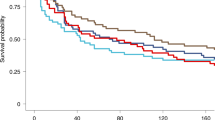
Extended Data Fig. 3 Protective efficacy of PfSPZ-CVac(CQ) and PfSPZ-CVac(PYR + CQ) against homologous PfSPZ CHMI in the PfSPZ-CVac low-dose study.
Participants were followed for 27 days after CHMI, and a survival curve is presented displaying the number of participants that remained protected throughout follow-up after homologous CHMI. To assess vaccine efficacy, the unvaccinated group was compared with the vaccine group for time to first detectable parasitaemia after the final challenge using a log-rank test, and the presence or absence of parasitaemia using a two-tailed Barnard’s test. In the pilot PfSPZ-CVac(PYR + CQ) group (to test safety), 0/2 participants were protected; in the main PfSPZ-CVac(PYR+CQ) group, 2/9 (22.2%, P = 0.8) participants were protected; in the PfSPZ-CVac(CQ) group, 4/5 (80%, P = 0.048; 95% confidence interval, 1–99%) participants were protected.
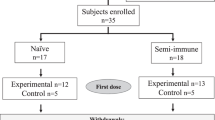
Extended Data Fig. 4 Trial profile for the PfSPZ-CVac high-dose study.
αOther, 15 withdrew consent, 6 deemed eligible after study was fully enrolled, 3 lost to follow-up, 2 schedule conflict, 1 withdrawn before randomization. βOne individual enrolled in the PYR group was withdrawn before receiving the first vaccination owing to acute illness, and enrolled in the control group. This person is counted twice. γBoth participants were withdrawn before receiving vaccination 1. δThe two serious adverse events in the CQ group were mental status change and pneumothorax.
Extended Data Fig. 5 Vδ2 γδ T cells at baseline in protected and infected individuals who received the vaccine.
Filled circles indicate vaccinated participants in the high-dose study, and open circles indicate vaccinated participants in the low-dose study. Median values are displayed and error bars indicate the IQR. For protected individuals in the high-dose study, n = 20; for protected individuals in the low-dose study, n = 6. For infected individuals in the high-dose study, n = 3; for infected individuals in the low-dose study, n = 8.
Extended Data Fig. 6 Anti-PfCSP IgM antibodies in vaccinated participants in the PfSPZ-CVac high-dose study analysed using ELISA.
IgM antibodies against PfCSP (net OD 1.0) 2 weeks after the third dose of PfSPZ-CVac(PYR) and PfSPZ-CVac(CQ); and immediately before CHMI. Median values are displayed and error bars indicate the IQR. At both time points, sample sizes for the PYR high-dose study, n = 14 for protected and n = 3 for infected vaccinated participants; for the CQ high-dose study, n = 6 for protected vaccinated individuals at 2 weeks after the third CVac and n = 5 for protected vaccinated individuals at before CHMI. No vaccinated participants were infected in the CQ high-dose study.
Extended Data Fig. 7 Antibody responses in vaccinated participants of the PfSPZ-CVac low-dose and PfSPZ-CVac high-dose studies analysed using automated immunofluorescence and automated inhibition of sporozoite invasion assays.
For each panel, filled circles are protected participants, open circles are infected participants who received homologous CHMI, filled triangles are uninfected (protected) participants and open triangles are infected participants who received heterologous CHMI. Median values are displayed and error bars indicate the IQR. P values were calculated using two-sided Wilcoxon–Mann–Whitney tests and group differences with P > 0.07 are not indicated. a, b, Antibodies against PfCSP by automated immunofluorescence assay were analysed 2 weeks after the third dose of PfSPZ-CVac(PYR) and PfSPZ-CVac(CQ) (a) and immediately before CHMI (b). c, d, Antibodies against PfCSP by automated inhibition of sporozoite invasion were analysed 2 weeks after the third dose of PfSPZ-CVac(PYR) and PfSPZ-CVac(CQ) (c) and immediately before CHMI (d). At both time points, sample sizes for the PYR low-dose study, n = 2 for protected and n = 7 for infected vaccinated participants; for the PYR high-dose study, n = 14 for protected and n = 3 for infected vaccinated participants; for the CQ low-dose study, n = 4 for protected and n = 1 for infected vaccinated participants; for the CQ high-dose study, n = 6 for protected participants in a and c (2 weeks after the third CVac), and n = 5 for protected participants in b and d (before CHMI). No vaccinated participants were infected in the CQ high-dose study. Anti-PfCSP antibodies at both 2 weeks after the last dose (high-dose 1,941 versus low-dose 382, P = 0.043) and immediately before CHMI (high-dose 1,149 versus low-dose 95, P = 0.009) were significantly higher in the high-dose PfSPZ-CVac(PYR) participants compared with the low-dose PfSPZ-CVac(PYR) participants. In PfPSZ-CVac(CQ) participants, the anti-PfSPZ antibodies were higher but not significant (2 weeks after the third dose, 300 versus 4,272, P = 0.176; before CHMI, 245 versus 910, P = 0.310). The inhibition of sporozoite invasion assay showed a statistically significant increase after vaccination for the high-dose PfSPZ-CVac(PYR) versus low-dose PfSPZ-CVac(PYR) when measured 2 weeks after the third dose (45.00 versus 3.81, P < 0.001) and immediately before CHMI (39.87 versus 1.00, P < 0.001). In PfPSZ-CVac(CQ) participants, the anti-PfCSP antibodies were significantly higher in the high-dose group when measured 2 weeks after the third dose (29.79 versus 8.79, P = 0.054) but not significantly different before CHMI (5.67 versus 7.32, P = 0.671).
Extended Data Fig. 8 Detection of parasitaemia by qPCR in each individual after the first, second and third dose of PfSPZ-CVac in the high-dose study.
Parasitaemia was measured in participants on days 6–10 after each PfSPZ-CVac dose. Vax, PfSPZ Challenge + CQ or PYR administration and follow-up until day 14 after PfSPZ Challenge 1 and day 10 after PfSPZ Challenge doses 2 and 3.
Extended Data Fig. 9 In vitro PYR activity against liver-stage parasites.
PYR was added to the cultures (blue) at three concentrations (1 μM, 10 μM and 100 μM), starting 2 days after infection and replaced with daily medium changes until day 4, at which point the cultures were fixed. The diameter of representative parasites of each group on day 4 are shown. Ctl, control. Statistical significance was determined by one-sided ANOVA. ***P < 0.001 (exact P = 0.0006); ****P < 0.0001; ns, not significant (exact P = 0.2335).
Extended Data Fig. 10 Flow cytometry gating strategy used to enumerate T cell subsets using flow cytometry ex vivo staining of whole blood.
The FSC/SSC parameters were used to define the lymphocyte gate as the first gate in the whole blood ex vivo assay. Within the lymphocyte gate, CD3-Alexa-700-positive events (T cell gate) were gated against CD56-PE-Cy7-positive events (NK cells). In the T cell gate, plotting Vδ2-FITC versus gdTCR-PE identified discrete Vδ2+, Vδ2− and CD3+γδTCR− populations. The T cell gate was used to further delineate CD4- and CD8-positive events.
Supplementary information
Supplementary Information
This file contains notes on the clinical trial design for the first low-dose study, Supplementary Methods and Results and Supplementary Tables 1-4.
Supplementary Data
Raw data showing Vd2 results.
Rights and permissions
About this article
Cite this article
Mwakingwe-Omari, A., Healy, S.A., Lane, J. et al. Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity. Nature 595, 289–294 (2021). https://doi.org/10.1038/s41586-021-03684-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03684-z
This article is cited by
-
Accelerated prime-and-trap vaccine regimen in mice using repRNA-based CSP malaria vaccine
npj Vaccines (2024)
-
Malaria blood stage infection suppresses liver stage infection via host-induced interferons but not hepcidin
Nature Communications (2024)
-
Profiling the antibody response of humans protected by immunization with Plasmodium vivax radiation-attenuated sporozoites
Scientific Reports (2024)
-
A replication competent Plasmodium falciparum parasite completely attenuated by dual gene deletion
EMBO Molecular Medicine (2024)
-
Protective efficacy and safety of radiation-attenuated and chemo-attenuated Plasmodium Falciparum sporozoite vaccines against controlled and natural malaria infection: a systematic review and meta-analysis of randomized controlled trials
Infection (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.