Abstract
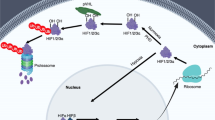
Hypoxia-inducible factors (HIFs), a family of transcription factors activated by hypoxia, consist of three α-subunits (HIF1α, HIF2α and HIF3α) and one β-subunit (HIF1β), which serves as a heterodimerization partner of the HIFα subunits. HIFα subunits are stabilized from constitutive degradation by hypoxia largely through lowering the activity of the oxygen-dependent prolyl hydroxylases that hydroxylate HIFα, leading to their proteolysis. HIF1α and HIF2α are expressed in different tissues and regulate target genes involved in angiogenesis, cell proliferation and inflammation, and their expression is associated with different disease states. HIFs have been widely studied because of their involvement in cancer, and HIF2α-specific inhibitors are being investigated in clinical trials for the treatment of kidney cancer. Although cancer has been the major focus of research on HIF, evidence has emerged that this pathway has a major role in the control of metabolism and influences metabolic diseases such as obesity, type 2 diabetes mellitus and non-alcoholic fatty liver disease. Notably increased HIF1α and HIF2α signalling in adipose tissue and small intestine, respectively, promotes metabolic diseases in diet-induced disease models. Inhibition of HIF1α and HIF2α decreases the adverse diet-induced metabolic phenotypes, suggesting that they could be drug targets for the treatment of metabolic diseases.
Key points
-
Obesity triggers hypoxia in adipose tissue and the small intestine, which stabilizes and activates hypoxia-inducible factor (HIF)1α and HIF2α signalling, resulting in adverse metabolic effects, including insulin resistance and non-alcoholic fatty liver disease.
-
Induction of HIF1α in adipocytes, through a suppressor of cytokine signalling 3 (SOCS3)–signal transducer and activator of transcription 3 (STAT3) axis, leads to the upregulation of inflammation and downregulation of adiponectin expression, resulting in insulin resistance.
-
Activation of HIF2α in the small intestine increases expression of sialidase 3, resulting in an elevation of small intestinal and serum levels of ceramides that in turn potentiate obesity-associated metabolic diseases.
-
Genetic or chemical inhibition of HIF1α and HIF2α signalling in adipose tissue and the small intestine ameliorates obesity-associated metabolic diseases, indicating that they could be targeted for treatment of metabolic disorders.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
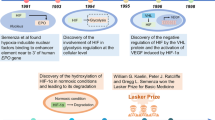
Semenza, G. L. & Wang, G. L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol. 12, 5447–5454 (1992).
Wang, G. L. & Semenza, G. L. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 268, 21513–21518 (1993).
Hoffman, E. C. et al. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science 252, 954–958 (1991).
Ravenna, L., Salvatori, L. & Russo, M. A. HIF3α: the little we know. FEBS J. 283, 993–1003 (2016).
Haase, V. H. HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial. Int. 21 (Suppl. 1), S110–S124 (2017).
Lisy, K. & Peet, D. J. Turn me on: regulating HIF transcriptional activity. Cell Death Differ. 15, 642–649 (2008).
Maxwell, P. H. et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999).
Ivan, M. et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Labrecque, M. P., Prefontaine, G. G. & Beischlag, T. V. The aryl hydrocarbon receptor nuclear translocator (ARNT) family of proteins: transcriptional modifiers with multi-functional protein interfaces. Curr. Mol. Med. 13, 1047–1065 (2013).
Nebert, D. W. Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog. Lipid Res. 67, 38–57 (2017).
Bersten, D. C., Sullivan, A. E., Peet, D. J. & Whitelaw, M. L. bHLH-PAS proteins in cancer. Nat. Rev. Cancer 13, 827–841 (2013).
Semenza, G. L. Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012).
Zhang, Y., Shao, Z., Zhai, Z., Shen, C. & Powell-Coffman, J. A. The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans. PLOS One 4, e6348 (2009).
Tian, H., Hammer, R. E., Matsumoto, A. M., Russell, D. W. & McKnight, S. L. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 12, 3320–3324 (1998).
Ramakrishnan, S. K. & Shah, Y. M. Role of intestinal HIF-2α in health and disease. Annu. Rev. Physiol. 78, 301–325 (2016).
Keith, B., Johnson, R. S. & Simon, M. C. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 12, 9–22 (2011).
Dengler, V. L., Galbraith, M. & Espinosa, J. M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 49, 1–15 (2014).
Liu, Y., Cox, S. R., Morita, T. & Kourembanas, S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5’ enhancer. Circ. Res. 77, 638–643 (1995).
Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 69 (Suppl. 3), 4–10 (2005).
Arjaans, M. et al. VEGF pathway targeting agents, vessel normalization and tumor drug uptake: from bench to bedside. Oncotarget 7, 21247–21258 (2016).
Wallace, E. M. et al. A small-molecule antagonist of HIF2α Is efficacious in preclinical models of renal cell carcinoma. Cancer Res. 76, 5491–5500 (2016).
Chen, W. et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016).
Martinez-Saez, O., Gajate Borau, P., Alonso-Gordoa, T., Molina-Cerrillo, J. & Grande, E. Targeting HIF-2α in clear cell renal cell carcinoma: A promising therapeutic strategy. Crit. Rev. Oncol. Hematol. 111, 117–123 (2017).
Ye, J., Gao, Z., Yin, J. & He, Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 293, E1118–1128 (2007).
Trayhurn, P., Wang, B. & Wood, I. S. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br. J. Nutr. 100, 227–235 (2008).
Pasarica, M. et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 (2009).
Lee, Y. S. et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014).
Regazzetti, C. et al. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes 58, 95–103 (2009).
Halberg, N. et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 29, 4467–4483 (2009).
Jun, J. C. et al. Adipose HIF-1α causes obesity by suppressing brown adipose tissue thermogenesis. J. Mol. Med. (Berl.) 95, 287–297 (2017).
Zhang, X. et al. Adipose tissue-specific inhibition of hypoxia-inducible factor 1α induces obesity and glucose intolerance by impeding energy expenditure in mice. J. Biol. Chem. 285, 32869–32877 (2010).
Basse, A. L. et al. Regulation of glycolysis in brown adipocytes by HIF-1α. Sci. Rep. 7, 4052 (2017).
Matsuura, H. et al. Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation 127, 2078–2087 (2013).
He, Q. et al. Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am. J. Physiol. Endocrinol. Metab. 300, E877–885 (2011).
Jiang, C. et al. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 60, 2484–2495 (2011).
Lee, K. Y., Gesta, S., Boucher, J., Wang, X. L. & Kahn, C. R.The differential role of Hif1β/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab. 14, 491–503 (2011).
Krishnan, J. et al. Dietary obesity-associated Hif1α activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev. 26, 259–270 (2012).
Lee, K. et al. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc. Natl Acad. Sci. USA 106, 17910–17915 (2009).
Jiang, C. et al. Hypoxia-inducible factor 1α regulates a SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes. J. Biol. Chem. 288, 3844–3857 (2013).
Sun, K., Halberg, N., Khan, M., Magalang, U. J. & Scherer, P. E. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell. Biol. 33, 904–917 (2013).
Kanatani, Y. et al. Effects of pioglitazone on suppressor of cytokine signaling 3 expression: potential mechanisms for its effects on insulin sensitivity and adiponectin expression. Diabetes 56, 795–803 (2007).
Zhang, S. Y. et al. Adipocyte-derived lysophosphatidylcholine activates adipocyte and adipose tissue macrophage nod-Like receptor protein 3 inflammasomes mediating homocysteine-induced insulin resistance. EBioMed 31, 202–216 (2018).
Li, Y. et al. Homocysteine upregulates resistin production from adipocytes in vivo and in vitro. Diabetes 57, 817–827 (2008).
Pang, Y. et al. Intermedin restores hyperhomocysteinemia-induced macrophage polarization and improves insulin resistance in mice. J. Biol. Chem. 291, 12336–12345 (2016).
Saltiel, A. R. & Olefsky, J. M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest. 127, 1–4 (2017).
Reilly, S. M. & Saltiel, A. R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 13, 633–643 (2017).
Guentsch, A. et al. PHD2 Is a regulator for glycolytic reprogramming in macrophages. Mol. Cell. Biol. 37, e00236–00216 (2017).
Wang, N., Liang, H. & Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front. Immunol. 5, 614 (2014).
Han, S. et al. Liver X receptor agonist therapy prevents diffuse alveolar hemorrhage in murine lupus by repolarizing macrophages. Front. Immunol. 9, 135 (2018).
Boutens, L. et al. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia 61, 942–953 (2018).
Takikawa, A. et al. HIF-1α in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes 65, 3649–3659 (2016).
Krishnan, J. et al. Activation of a HIF1α-PPARγ axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 9, 512–524 (2009).
Lu, H., Gao, Z., Zhao, Z., Weng, J. & Ye, J. Transient hypoxia reprograms differentiating adipocytes for enhanced insulin sensitivity and triglyceride accumulation. Int. J. Obes. (Lond.) 40, 121–128 (2016).
Gunton, J. E. et al. Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122, 337–349 (2005).
Cheng, K. et al. Hypoxia-inducible factor-1α regulates βcell function in mouse and human islets. J. Clin. Invest. 120, 2171–2183 (2010).
Stokes, R. A. et al. Hypoxia-inducible factor-1α (HIF-1α) potentiates β-cell survival after islet transplantation of human and mouse islets. Cell Transplant. 22, 253–266 (2013).
Zehetner, J. et al. PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic β cells. Genes Dev. 22, 3135–3146 (2008).
Cantley, J. et al. Deletion of the von hippel-lindau gene in pancreatic β cells impairs glucose homeostasis in mice. J. Clin. Invest. 119, 125–135 (2009).
Zelzer, E. et al. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J. 17, 5085–5094 (1998).
Kim, J. W., Tchernyshyov, I., Semenza, G. L. & Dang, C. V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006).
Gorden, D. L. et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J. Lipid Res. 56, 722–736 (2015).
Kucejova, B. et al. Uncoupling hypoxia signaling from oxygen sensing in the liver results in hypoketotic hypoglycemic death. Oncogene 30, 2147–2160 (2011).
Luo, B. et al. Hepatic PHD2/HIF-1α axis is involved in postexercise systemic energy homeostasis. FASEB J. https://doi.org/10.1096/fj.201701139R (2018).
Minamishima, Y. A. et al. A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol. Cell. Biol. 29, 5729–5741 (2009).
Nath, B. et al. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology 53, 1526–1537 (2011).
Nishiyama, Y. et al. HIF-1α induction suppresses excessive lipid accumulation in alcoholic fatty liver in mice. J. Hepatol. 56, 441–447 (2012).
Ochiai, D. et al. Disruption of HIF-1α in hepatocytes impairs glucose metabolism in diet-induced obesity mice. Biochem. Biophys. Res. Commun. 415, 445–449 (2011).
Scott, C. H. et al. Hepatic aryl hydrocarbon receptor nuclear translocator (ARNT) regulates metabolism in mice. PLOS One 12, e0186543 (2017).
Ouyang, X. et al. Digoxin suppresses pyruvate kinase M2-promoted HIF-1α transactivation in steatohepatitis. Cell Metab. 27, 339–350.e3 (2018).
Lee, T. Y., Leu, Y. L. & Wen, C. K. Modulation of HIF-1α and STAT3 signaling contributes to anti-angiogenic effect of YC-1 in mice with liver fibrosis. Oncotarget 8, 86206–86216 (2017).
Hwang, S. et al. Hypoxia-inducible factor 1α activates insulin-induced gene 2 (insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J. Biol. Chem. 292, 9382–9393 (2017).
Ramakrishnan, S. K. & Shah, Y. M. A central role for hypoxia-inducible factor (HIF)-2α in hepatic glucose homeostasis. Nutr. Healthy Aging 4, 207–216 (2017).
Rankin, E. B. et al. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol. Cell. Biol. 29, 4527–4538 (2009).
Rahtu-Korpela, L. et al. Hypoxia-inducible factor prolyl 4-hydroxylase-2 inhibition protects against development of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 36, 608–617 (2016).
Qu, A. et al. Hypoxia-inducible transcription factor 2α promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology 54, 472–483 (2011).
Li, D. et al. Hepatic hypoxia-inducible factors inhibit PPARα expression to exacerbate acetaminophen induced oxidative stress and hepatotoxicity. Free Radic. Biol. Med. 110, 102–116 (2017).
Patterson, A. D., Shah, Y. M., Matsubara, T., Krausz, K. W. & Gonzalez, F. J. Peroxisome proliferator-activated receptor α induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology 56, 281–290 (2012).
Kersten, S. Angiopoietin-like 3 in lipoprotein metabolism. Nat. Rev. Endocrinol. 13, 731–739 (2017).
Morello, E. et al. Hypoxia-inducible factor 2α drives nonalcoholic fatty liver progression by triggering hepatocyte release of histidine-rich glycoprotein. Hepatology 67, 2196–2214 (2018).
Bartneck, M. et al. Histidine-rich glycoprotein promotes macrophage activation and inflammation in chronic liver disease. Hepatology 63, 1310–1324 (2016).
Ramakrishnan, S. K. et al. HIF2α Is an essential molecular brake for postprandial hepatic glucagon response independent of insulin signaling. Cell Metab. 23, 505–516 (2016).
Taniguchi, C. M. et al. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat. Med. 19, 1325–1330 (2013).
Wei, K. et al. A liver Hif-2α-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nat. Med. 19, 1331–1337 (2013).
Singh, V. et al. Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab. 22, 983–996 (2015).
Jiang, C. et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest. 125, 386–402 (2015).
Gonzalez, F. J., Jiang, C. & Patterson, A. D. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 51, 845–859 (2016).
Xie, C. et al. Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat. Med. 23, 1298–1308 (2017).
Walter, K. M. et al. Hif-2α promotes degradation of mammalian peroxisomes by selective autophagy. Cell Metab. 20, 882–897 (2014).
Schonenberger, M. J., Krek, W. & Kovacs, W. J. EPAS1/HIF-2α is a driver of mammalian pexophagy. Autophagy 11, 967–969 (2015).
Rahtu-Korpela, L. et al. HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes 63, 3324–3333 (2014).
Garcia-Martin, R. et al. Adipocyte-specific hypoxia-inducible factor 2α deficiency exacerbates obesity-induced brown adipose tissue dysfunction and metabolic dysregulation. Mol. Cell. Biol. 36, 376–393 (2015).
Park, J. et al. VEGF-A-expressing adipose tissue shows rapid beiging and enhanced survival after transplantation and confers IL-4-Iidependent metabolic improvements. Diabetes 66, 1479–1490 (2017).
Kim, K. H. et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res. 27, 1309–1326 (2017).
Qiu, Y. et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157, 1292–1308 (2014).
Takeda, N. et al. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev. 24, 491–501 (2010).
Dehn, S. et al. HIF-2α in resting macrophages tempers mitochondrial reactive oxygen species to selectively repress MARCO-dependent phagocytosis. J. Immunol. 197, 3639–3649 (2016).
Semenza, G. L. & Prabhakar, N. R. The role of hypoxia-inducible factors in carotid body (patho) physiology. J. Physiol. https://doi.org/10.1113/JP275696 (2018).
Yuan, G. et al. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J. Cell. Physiol. 226, 2925–2933 (2011).
Nanduri, J. et al. Intermittent hypoxia degrades HIF-2α via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc. Natl Acad. Sci. USA 106, 1199–1204 (2009).
Mastrogiannaki, M. et al. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J. Clin. Invest. 119, 1159–1166 (2009).
Pinhas-Hamiel, O. et al. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int. J. Obes. Relat. Metab. Disord. 27, 416–418 (2003).
Shah, Y. M., Matsubara, T., Ito, S., Yim, S. H. & Gonzalez, F. J. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 9, 152–164 (2009).
Yanoff, L. B. et al. Inflammation and iron deficiency in the hypoferremia of obesity. Int. J. Obes. (Lond.) 31, 1412–1419 (2007).
Cepeda-Lopez, A. C. et al. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am. J. Clin. Nutr. 93, 975–983 (2011).
Simcox, J. A. & McClain, D. A. Iron and diabetes risk. Cell Metab. 17, 329–341 (2013).
Safran, M. et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc. Natl Acad. Sci. USA 103, 105–110 (2006).
Scheuermann, T. H. et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat. Chem. Biol. 9, 271–276 (2013).
Miyagi, T., Wada, T., Yamaguchi, K., Hata, K. & Shiozaki, K. Plasma membrane-associated sialidase as a crucial regulator of transmembrane signalling. J. Biochem. 144, 279–285 (2008).
Xie, C. et al. Metabolic profiling of the novel HIF2α inhibitor PT2385 in vivo and in vitro. Drug Metab. Dispos. 46, 336–345 (2018).
Chavez, J. A. & Summers, S. A. A ceramide-centric view of insulin resistance. Cell Metab. 15, 585–594 (2012).
Chaurasia, B. et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 24, 820–834 (2016).
Summers, S. A. & Goodpaster, B. H. CrossTalk proposal: Intramyocellular ceramide accumulation does modulate insulin resistance. J. Physiol. 594, 3167–3170 (2016).
Summers, S. A. Could ceramides become the new cholesterol? Cell Metab. 27, 276–280 (2018).
Matsubara, T., Li, F. & Gonzalez, F. J. FXR signaling in the enterohepatic system. Mol. Cell Endocrinol. 368, 17–29 (2013).
Khavandgar, Z. & Murshed, M. Sphingolipid metabolism and its role in the skeletal tissues. Cell. Mol. Life Sci. 72, 959–969 (2015).
Li, F. et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 4, 2384 (2013).
Xie, C. et al. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66, 613–626 (2017).
Tomita, S., Sinal, C. J., Yim, S. H. & Gonzalez, F. J. Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1α. Mol. Endocrinol. 14, 1674–1681 (2000).
Wang, X. L. et al. Ablation of ARNT/HIF1β in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 9, 428–439 (2009).
Girer, N. G., Murray, I. A., Omiecinski, C. J. & Perdew, G. H. Hepatic aryl hydrocarbon receptor attenuates fibroblast growth factor 21 expression. J. Biol. Chem. 291, 15378–15387 (2016).
Bhattarai, D., Xu, X. & Lee, K. Hypoxia-inducible factor-1 (HIF-1) inhibitors from the last decade: a “structure-activity relationship” perspective. Med. Res. Rev. 38, 1404–1442 (2017).
Lee, K. et al. Identification of malate dehydrogenase 2 as a target protein of the HIF-1 inhibitor LW6 using chemical probes. Angew. Chem. Int. Ed Engl. 52, 10286–10289 (2013).
Ban, H. S. et al. A novel malate dehydrogenase 2 inhibitor suppresses hypoxia-inducible factor-1 by regulating mitochondrial respiration. PLOS One 11, e0162568 (2016).
Eleftheriadis, T., Pissas, G., Antoniadi, G., Liakopoulos, V. & Stefanidis, I. Malate dehydrogenase-2 inhibitor LW6 promotes metabolic adaptations and reduces proliferation and apoptosis in activated human T cells. Exp. Ther. Med. 10, 1959–1966 (2015).
Yin, S. et al. Arylsulfonamide KCN1 inhibits in vivo glioma growth and interferes with HIF signaling by disrupting HIF-1α interaction with cofactors p300/CBP. Clin. Cancer Res. 18, 6623–6633 (2012).
Wang, W. et al. KCN1, a novel synthetic sulfonamide anticancer agent: in vitro and in vivo anti-pancreatic cancer activities and preclinical pharmacology. PLOS One 7, e44883 (2012).
Zhao, T. et al. HIF-1-mediated metabolic reprogramming reduces ROS levels and facilitates the metastatic colonization of cancers in lungs. Sci. Rep. 4, 3793 (2014).
Tsui, L., Chang, S. F., Huang, H. P., Fong, T. H. & Wang, I. J. YC-1 induces lipid droplet formation in RAW 264.7 macrophages. J. Biomed. Sci. 23, 2 (2016).
Chin, C. H. et al. YC-1, a potent antithrombotic agent, induces lipolysis through the PKA pathway in rat visceral fat cells. Eur. J. Pharmacol. 689, 1–7 (2012).
Hung, C. C. & Liou, H. H. YC-1, a novel potential anticancer agent, inhibit multidrug-resistant protein via cGMP-dependent pathway. Invest. New Drugs 29, 1337–1346 (2011).
Pugh, C. W. Modulation of the hypoxic response. Adv. Exp. Med. Biol. 903, 259–271 (2016).
Acknowledgements
The authors acknowledge the support of the National Cancer Institute Intramural Research Program, the NIH, the National Key Research and Development Program of China (2016YFC0903100), the National Natural Science Foundation of the People’s Republic of China (81522007, 81470554 and 31401011) and the Fundamental Research Funds for the Central Universities: Clinical Medicine Plus X–Young Scholars Project of Peking University (PKU2018LCXQ013).
Author information
Authors and Affiliations
Contributions
F.J.G. and C.J. researched the data for the article, contributed to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission. C.X. reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gonzalez, F.J., Xie, C. & Jiang, C. The role of hypoxia-inducible factors in metabolic diseases. Nat Rev Endocrinol 15, 21–32 (2019). https://doi.org/10.1038/s41574-018-0096-z
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-018-0096-z
This article is cited by
-
PDGFRβ + cell HIF2α is dispensable for white adipose tissue metabolic remodeling and hepatic lipid accumulation in obese mice
Lipids in Health and Disease (2024)
-
Hypoxia enhances autophagy level of human sperms
Scientific Reports (2024)
-
Cheong-sang-gyeon-tong-tang improves hepatic steatosis by regulating cholesterol metabolism
Molecular & Cellular Toxicology (2024)
-
Diphenyl Diselenide Attenuates Mitochondrial Damage During Initial Hypoxia and Enhances Resistance to Recurrent Hypoxia
Neurotoxicity Research (2024)
-
The therapeutic potential for targeting CSE/H2S signaling in macrophages against Escherichia coli infection
Veterinary Research (2023)