Abstract
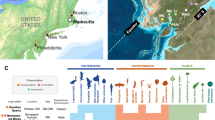
Reconstruction of palaeobiomes, ancient communities that exhibit a physiognomic and functional structure controlled by their environment, depends on proxies from different disciplines. Based on terrestrial mammal fossils, the late Miocene vegetation from China to the eastern Mediterranean and East Africa has been reconstructed as a single cohesive biome with increasingly arid conditions, with modern African savannahs the surviving remnant. Here, we test this reconstruction using plant fossils spanning 14–4 million years ago from sites in Greece, Bulgaria, Turkey, the Tian Shan Mountains and Baode County in China, and East Africa. The western Eurasian sites had a continuous forest cover of deciduous or evergreen angiosperms and gymnosperms, with 15% of 1,602 fossil occurrences representing conifers, which were present at all but one of the sites. Raup–Crick analyses reveal high floristic similarity between coeval eastern Mediterranean and Chinese sites, and low similarity between Eurasian and African sites. The disagreement between plant-based reconstructions, which imply that late Miocene western Eurasia was covered by evergreen needleleaf forests and mixed forests, and mammal-based reconstructions, which imply a savannah biome, throws into doubt the approach of inferring Miocene precipitation and open savannah habitats solely from mammalian dental traits. Organismal communities are constantly changing in their species composition, and neither animal nor plant traits by themselves are sufficient to infer entire ancient biomes. The plant fossil record, however, unambiguously rejects the existence of a cohesive savannah biome from eastern Asia to northeast Africa.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available in the supplementary tables.
References
Solounias, N. & Dawson-Saunders, B. Dietary adaptations and paleoecology of the late Miocene ruminants from Pikermi and Samos in Greece. Palaeogeogr. Palaeoclimatol. Palaeoecol. 65, 149–172 (1988).
Bernor, R. L., Solounias, N., Swisher, C. C. III & van Couvering, J. A. in The Evolution of Western Eurasian Neogene Mammal Faunas (eds Bernor, R. L., Fahlbusch, V. & Mittmann, H.-W.) 137–154 (Columbia Univ. Press, New York, 1996).
Fortelius, M. et al. in The Evolution of Western Eurasian Neogene Mammal Faunas (eds Bernor, R. L., Fahlbusch, V. & Mittmann, H.-W.) 414–448 (Columbia Univ. Press, New York, 1996).
Eronen, J. T. et al. Distribution history and climatic controls of the late Miocene Pikermian chronofauna. Proc. Natl Acad. Sci. USA 106, 11867–11871 (2009).
Solounias, N., Rivals, F. & Semprebon, G. M. Dietary interpretation and paleoecology of herbivores from Pikermi and Samos (late Miocene of Greece). Paleobiology 36, 113–136 (2010).
Bernor, R. L., Andrews, P. J., Solounias, N. & van Couvering, J. A. H. The evolution of ‘Pontian’ mammal faunas: some zoogeographic, paleoecologic and chronostratigraphic considerations. Ann. Géol. Pays Hellén. Hors Sér. 1, 81–89 (1979).
Solounias, N., Plavcan, J. M., Quade, J. & Witmer, L. in The Evolution of Neogene Terrestrial Ecosystems in Europe (eds Agustí, J., Rook, L. & Andrews, P.) 436–453 (Cambridge Univ. Press, Cambridge, 1999).
Jernvall, J. & Fortelius, M. Common mammals drive the evolutionary increase of hypsodonty in the Neogene. Nature 417, 538–540 (2002).
Fortelius, M. et al. Fossil mammals resolve regional patterns of Eurasian climate change over 20 million years. Evol. Ecol. Res. 4, 1005–1016 (2002).
Kaya, F. et al. The rise and fall of the Old-World savannah fauna and the origins of the African savannah biome. Nat. Ecol. Evol. 2, 241–246 (2018).
Bernor, R. L. et al. Systematic, stratigraphic, and paleoenvironmental contexts of first-appearing Hipparion in the Vienna Basin, Austria. J. Vertebr. Paleontol. 8, 427–452 (1988).
Raup, D. & Crick, R. E. Measurement of faunal similarity in paleontology. J. Paleontol. 53, 1213–1227 (1979).
Chase, J. M., Kraft, N. J. B., Smith, K. G., Vellend, M. & Inouye, B. D. Using null models to disentangle variation in community dissimilarity from variation in α-diversity. Ecosphere 2, art24 (2011).
Harzhauser, M. & Piller, W. E. Benchmark data of a changing sea—palaeogeography, palaeobiogeography and events in the central Paratethys during the Miocene. Palaeogeogr. Palaeoclimatol. Palaeoecol. 253, 8–31 (2007).
Cohen, K. M., Finney, S. C., Gibbard, P. L. & Fan, J.-X. The ICS international chronostratigraphic chart. Episodes 36, 199–204 (2013).
Woodward, F. I., Lomas, M. R. & Kelly, C. K. Global climate and the distribution of plant biomes. Phil. Trans. R. Soc. Lond. B 359, 1465–1476 (2004).
Olson, D. M. et al. Terrestrial ecosystems of the world: a new map of life on Earth. Bioscience 51, 933–938 (2001).
Kovar-Eder, J. Pannonian (upper Miocene) vegetational character and climatic inferences in the central Paratethys area. Ann. Naturhist. Mus. Wien 88, 117–129 (1987).
Kovar-Eder, J., Kvaček, Z., Martinetto, E. & Roiron, P. Late Miocene to early Pliocene vegetation of southern Europe (7–4 Ma) as reflected in the megafossil plant record. Palaeogeogr. Palaeoclimatol. Palaeoecol. 238, 321–339 (2006).
Ivanov, D. et al. Miocene vegetation and climate dynamics in eastern and central Paratethys (southeastern Europe). Palaeogeogr. Palaeoclimatol. Palaeoecol. 304, 262–275 (2011).
Draxner-Höck, G., Harzhauser, M. & Göhlich, U. B. Fossil record and dynamics of late Miocene small mammal faunas of the Vienna Basin and adjacent basins, Austria. C. R. Palevol. 15, 855–862 (2016).
Bouchal, J. M. et al. Miocene palynofloras of the Tınaz lignite mine, Muğla, southwest Anatolia: taxonomy, palaeoecology and local vegetation change. Rev. Palaeobot. Palyno. 243, 1–36 (2017).
Syabryaj, S., Utescher, T., Molchanoff, S. & Bruch, A. A. Vegetation and palaeoclimate in the Miocene of Ukraine. Palaeogeography, Palaeoclimatology, Palaeoecology 253, 153–168 (2007).
Merceron, G., Novello, A. & Scott, R. S. Paleoenvironments inferred from phytoliths and dental microwear texture analyses of meso-herbivores. Geobios 49, 135–146 (2016).
Velitzelos, D., Bouchal, J. M. & Denk, T. Review of the Cenozoic floras and vegetation of Greece. Rev. Palaeobot. Palyno. 204, 56–117 (2014).
Denk, T., Güner, H. T. & Grimm, G. W. From mesic to arid: leaf epidermal features suggest preadaptation in Miocene dragon trees (Dracaena). Rev. Palaeobot. Palyno. 200, 211–228 (2014).
Bertini, A. & Martinetto, E. Messinian to Zanclean vegetation and climate of northern and central Italy. B. Soc. Paleontol. Ital. 47, 105–121 (2008).
Kurtén, B. The Chinese Hipparion fauna. A quantitative survey with comments on the ecology of the machairodonts and hyaenids and the taxonomy of the gazelles. Comment. Biol. 13, 1–82 (1952).
Kovar-Eder, J. & Kvaček, Z. The integrated plant record (IPR) to reconstruct Neogene vegetation: the IPR-vegetation analysis. Palaios 23, 97–111 (2007).
Jiang, H. & Ding, Z. A 20 Ma pollen record of East Asian summer monsoon evolution from Guyuan, Ningxia, China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 265, 30–38 (2008).
Bosboom, R. et al. Linking Tarim Basin sea retreat (West China) and Asian aridification in the late Eocene. Basin Res. 26, 621–640 (2014).
Quade, J., Solounias, N. & Cerling, T. E. Stable isotopic evidence from paleosol carbonates and fossil teeth in Greece for forest or woodlands over the past 11 Ma. Palaeogeogr. Palaeoclimatol. Palaeoecol. 108, 41–53 (1994).
Kayseri-Özer, M. S. et al. Palaeoclimatic and palaeo-environmental interpretations of the late Oligocene, late Miocene–early Pliocene in the Çankiri-Çorum Basin. Palaeogeogr. Palaeoclimatol. Palaeoecol. 467, 16–36 (2017).
Spassov, N., Böhme, M., Geraads, D., Kötter, S. & van Baak C. Pikermian mammal event, post-Pikermian mammal turnover and appearance of Graecopithecus. In 15th Congress of the Regional Committee on Mediterranean Neogene Stratigraphy, Book of Abstracts, 29 (RCMNS, 2017); http://www.rcmns2017.com/files/pdf/BOOK-OF-ABSTRACTS.pdf
Böhme, M. et al. Messinian age and savannah environment of the possible hominin Graecopithecus from Europe. PLoS ONE 12, e0177347 (2017).
Urban, M. A. et al. Isotopic evidence of C4 grasses in southwestern Europe during the early Oligocene–middle Miocene. Geology 38, 1091–1094 (2010).
Urban, M. A., Nelson, D. M., Jiménez-Moreno, G. & Hu, F. S. Carbon isotope analyses reveal relatively high abundance of C4 grasses during early–middle Miocene in southwestern Europe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 443, 10–17 (2016).
Passey, B. H., Eronen, J. T., Fortelius, M. & Zhang, Z. Paleodiets and paleoenvironments of late Miocene gazelles from north China: evidence from stable carbon isotopes. Vertebrat. Palasiatic. 45, 118–127 (2007).
Zhang, C. et al. C4 expansion in the central Inner Mongolia during the latest Miocene and early Pliocene. Earth Planet Sc. Lett. 287, 311–319 (2009).
Bernor, R. L., Ataabadi, M. M., Meshida, K. & Wolf, D. The Maragheh hipparions, late Miocene of Azarbaijan, Iran. Palaeobio. Palaeoenv. 96, 453–488 (2016).
Barry, J. C. et al. Faunal and environmental change in the Late Miocene Siwaliks of northern Pakistan. Paleobiology 28, 1–71 (2002).
Feakins, S. J., Levin, N. E., Liddy, H. M., Sieracki, A., Eglinton, T. I. & Bonnefille, R. Northeast African vegetation change over 12 m.y. Geology 41, 295–298 (2013).
Flynn, L. J., Pilbeam, D., Barry, J. C. & Morgan, M. E. Siwalik synopsis: a long stratigraphic sequence for the later Cenozoic of South Asia. C. R. Palevol. 15, 877–887 (2016).
Clements, F. E. The development and structure of biotic communities. J. Ecol. 5, 120–121 (1917).
Egerton, F. N. History of ecological sciences, part 59: niches, biomes, ecosystems, and systems. Bull. Ecol. Soc. Am. 98, 298–337 (2017).
Bastin, J.-F. et al. The extent of forest in dryland biomes. Science 356, 635–638 (2017).
Parr, C. L., Lehmann, C. E. R., Bond, W. J., Hoffmann, W. A. & Andersen, A. N. Tropical grassy biomes: misunderstood, neglected, and under threat. Trends Ecol. Evol. 29, 205–213 (2014).
Ratnam, J. et al. When is a ‘forest’ a savanna, and why does it matter? Glob. Ecol. Biogeogr. 20, 653–660 (2011).
Ratnam, J., Tomlinson, K. W., Rasquinha, D. N. & Sankaran, M. Savannahs of Asia: antiquity, biogeography, and an uncertain future. Phil. Trans. R. Soc. B 371, 20150305 (2016).
Lehmann, C. E., Archibald, S. A., Hoffmann, W. A. & Bond, W. J. Deciphering the distribution of the savanna biome. New Phytol. 191, 197–209 (2011).
Griffith, D. M. et al. Comment on ‘The extent of forest in dryland biomes’. Science 358, eaao1309 (2017).
Whittaker, R. H. Communities and Ecosystems 2nd edn (Macmillan, New York, 1975).
Gleason, H. A. The individualistic concept of the plant association. Torrey Botanical Club Bull. 53, 7–26 (1926).
Ricklefs, R. E. History and diversity: explorations at the intersection of ecology and evolution. Am. Nat. 170, S56–S70 (2007).
Ricklefs, R. E. Disintegration of the ecological community. Am. Nat. 172, 741–750 (2008).
Hayek, L.-A. C., Bernor, R. L., Solounias, N. & Steigerwald, P. Preliminary studies of hipparionine horse diet as measured by tooth microwear. Ann. Zool. Fenn. 28, 187–200 (1992).
Kaiser, T. M., Solounias, N., Fortelius, M., Bernor, R. & Schrenk, F. Tooth mesowear analysis on Hippotherium primigenium from the Vallesian Dinotheriensande (Germany)—a blind test study. Carolinea 58, 103–114 (2000).
Terrestrial Ecoregions (WWF, 2018); https://www.worldwildlife.org/biome-categories/terrestrial-ecoregions
Walter, H. & Breckle, S.-W. Ökologie der Erde. 3. Spezielle Ökologie der gemäßigten und Arktischen Zonen Euro-Nordasiens (Gustav Fischer Verlag, Stuttgart, 1986).
Woldring, H. & Cappers, R. The origin of the ‘wild orchards’ of Central Anatolia. Turk. J. Bot. 25, 1–9 (2001).
Bremond, L., Alexandre, A., Véla, E. & Guiot, J. Advantages and disadvantages of phytolith analysis for the reconstruction of Mediterranean vegetation: an assessment based on modern phytolith, pollen and botanical data (Luberon, France). Rev. Palaeobot. Palyno. 129, 213–228 (2004).
Lisitsyna, O. V., Giesecke, T. & Hicks, S. Exploring pollen percentage threshold values as an indicator for the regional presence of major European trees. Rev. Palaeobot. Palyno. 166, 311–324 (2011).
Salzmann, U. Are modern savannas degraded forests? A Holocene pollen record from the Sudanian vegetation zone of NE Nigeria. Veg. Hist. Archaebot. 9, 1–15 (2000).
Zhou, Y. et al. Vascular flora of Kenya, based on the flora of tropical East Africa. PhytoKeys 90, 113–126 (2017).
Van Zeist, W., Woldring, H. & Stapert, D. Late Quaternary vegetation and climate of southwestern Turkey. Palaeohistoria 17, 53–143 (1975).
Sirenko, E. A. Microrhythms in the evolution of Pliocene and early Pleistocene vegetation in eastern Ukraine. Paleontol. J. 34(suppl.1), S81–S86 (2000).
Qin, F. et al. Utility of surface pollen assemblages to delimit eastern Eurasian steppe types. PLoS ONE 10, e0119412 (2015).
Strömberg, C. A. E., Werdelin, L., Friis, E. M. & Saraç, G. The spread of grass-dominated habitats in Turkey and surrounding areas during the Cenozoic: phytolith evidence. Palaeogeogr. Palaeoclimatol. Palaeoecol. 250, 18–49 (2007).
Peel, M. C., Finlayson, B. L. & McMahon, T. A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 11, 1633–1644 (2007).
Acknowledgements
This work was supported by a Swedish Research Council grant to T.D. and an Austrian Science Fund Grant M 1751 (to G.W.G.). We thank F. Kaya for discussion of his Raup–Crick analyses and the shortcomings of different proxies for inferring past biomes.
Author information
Authors and Affiliations
Contributions
T.D. designed the initial study and performed analyses. C.M.Z. performed Raup–Crick analyses. G.W.G. performed Köppen climate analyses and designed most figures. S.S.R. provided conceptual input and wrote the first draft. All authors co-wrote the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures.
Supplementary Table 1
Western Eurasian plant fossil localities considered for this study. Location, type of floral assemblage (macrofossil, leaf; macrofossil, fruit; seed; microfossil, pollen and spores), Neogene mammal zone, age inference and full references.
Supplementary Table 2
Taxon lists and vegetation unit scoring for the Western Eurasian plant localities associated with mammal faunas in Greece, Bulgaria and Turkey. Also included are phytolith counts from Strömberg et al. (2007) as shown in Supplementary Fig. 2.
Supplementary Table 3
Taxon lists (genus, family levels) of plant assemblages from western Eurasia, Northeast Asia and East Africa spanning MN7+8–MN14 used for calculating Raup–Crick similarity indices.
Rights and permissions
About this article
Cite this article
Denk, T., Zohner, C.M., Grimm, G.W. et al. Plant fossils reveal major biomes occupied by the late Miocene Old-World Pikermian fauna. Nat Ecol Evol 2, 1864–1870 (2018). https://doi.org/10.1038/s41559-018-0695-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-018-0695-z
This article is cited by
-
Bearing Fruit: Miocene Apes and Rosaceous Fruit Evolution
Biological Theory (2023)
-
New evidence for the unique coexistence of two subfamilies of clawed perissodactyls (Mammalia, Chalicotheriidae) in the Upper Miocene of Romania and the Eastern Mediterranean
Journal of Mammalian Evolution (2023)
-
The nature of the Old World savannah palaeobiome
Nature Ecology & Evolution (2019)
-
Reconciling the stratigraphy and depositional history of the Lycian orogen-top basins, SW Anatolia
Palaeobiodiversity and Palaeoenvironments (2019)