Abstract
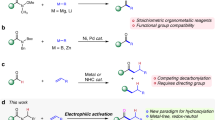
Alkenes are among the most versatile building blocks and are widely used for the production of polymers, detergents and synthetic lubricants. Currently, alkenes are sourced from petroleum feedstocks such as naphtha. In light of the necessity to invent sustainable production methods, multiple approaches to making alkenes from abundant fatty acids have been evaluated. However, all attempts so far have required at least one stoichiometric additive, which is an obstruction for applications at larger scales. Here, we report an approach to making olefins from carboxylic acids, in which every additional reaction constituent can be used as a catalyst. We show how abundant fatty acids can be converted to alpha-olefins, and expand the method to include structurally complex carboxylic acids, giving access to synthetically versatile intermediates. Our approach is enabled by the cooperative interplay between a cobalt catalyst, which functions as a proton reduction catalyst, and a photoredox catalyst, which mediates oxidative decarboxylation; coupling both processes enables catalytic conversion of carboxylic acids to olefins.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Crystallographic data for structure 1 reported in this article have been deposited at the Cambridge Crystallographic Data Centre under deposition number 1831368. Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif. All other data supporting the findings of this study are available within the article and its Supplementary Information, or from the corresponding author upon reasonable request.
References
Lappin, G. R. & Sauer, J. D. Alpha Olefins Applications Handbook (Marcel Dekker, New York, 1989).
Grubbs, R. H. in Handbook of Metathesis Vol. 2 (ed. Grubbs, R. H.) 1–4 (Wiley, Weinheim, 2003).
Horváth, I. T. Introduction: sustainable chemistry. Chem. Rev. 118, 369–371 (2018).
Miller, J. A., Nelson, J. A. & Byrne, M. P. A highly catalytic and selective conversion of carboxylic acids to 1-alkenes of one less carbon atom. J. Org. Chem. 58, 18–20 (1993).
Gooßen, L. J. & Rodríguez, N. A mild and efficient protocol for the conversion of carboxylic acids to olefins by a catalytic decarbonylative elimination reaction. Chem. Commun. 724–725 (2004)..
Maetani, S., Fukuyama, T., Suzuki, N., Ishihara, D. & Ryu, I. Efficient iridium-catalyzed decarbonylation reaction of aliphatic carboxylic acids leading to internal or terminal alkenes. Organometallics 30, 1389–1394 (2011).
Maetani, S., Fukuyama, T., Suzuki, N., Ishiharab, D. & Ryu, I. Iron-catalyzed decarbonylation reaction of aliphatic carboxylic acids leading to α-olefins. Chem. Commun. 48, 2552–2554 (2012).
John, A. et al. Nickel catalysts for the dehydrative decarbonylation of carboxylic acids to alkenes. Organometallics 35, 2391–2400 (2016).
John, A., Hillmyer, M. A. & Tolman, W. B. Anhydride-additive-free nickel-catalyzed deoxygenation of carboxylic acids to olefins. Organometallics 36, 506–509 (2017).
Chatterjee, A. & Jensen, V. R. A heterogeneous catalyst for the transformation of fatty acids to α-olefins. ACS Catal. 7, 2543–2547 (2017).
Edwards, J. T. et al. Decarboxylative alkenylation. Nature 545, 213–218 (2017).
Li, C. et al. Decarboxylative borylation. Science 356, eaam7355 (2017).
Fawcett, A. et al. Photoinduced decarboxylative borylation of carboxylic acids. Science 357, 283–286 (2017).
Tlahuext-Aca, A., Candish, L., Garza-Sanchez, R. A. & Glorius, F. Decarboxylative olefination of activated aliphatic acids enabled by dual organophotoredox/copper catalysis. ACS Catal. 8, 1715–1719 (2018).
Grant, J. L., Hsieh, C. H. & Makris, T. M. Decarboxylation of fatty acids to terminal alkenes by cytochrome P450 compound I. J. Am. Chem. Soc. 137, 4940–4943 (2015).
Dennig, A. et al. Oxidative decarboxylation of short-chain fatty acids to 1-alkenes. Angew. Chem. Int. Ed. 54, 8819–8822 (2015).
Bacha, J. D. & Kochi, J. K. Alkenes from acids by oxidative decarboxylation. Tetrahedron 24, 2215–2226 (1968).
Lande, S. S. & Kochi, J. K. Formation and oxidation of alkyl radicals by cobalt (iii) complexes. J. Am. Chem. Soc. 90, 5196–5207 (1968).
Anderson, J. M. & Kochi, J. K. Silver(i)-catalyzed oxidative decarboxylation of acids by peroxydisulfate. The role of silver(ii). J. Am. Chem. Soc. 92, 1651–1659 (1970).
Du, P. & Eisenberg, R. Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: recent progress and future challenges. Energy Environ. Sci. 5, 6012–6021 (2012).
Artero, V., Chavarot-Kerlidou, M. & Fontecave, M. Splitting water with cobalt. Angew. Chem. Int. Ed. 50, 7238–7266 (2011).
Dempsey, J. L., Brunschwig, B. S., Winkler, J. R. & Gray, H. B. Hydrogen evolution catalyzed by cobaloximes. Acc. Chem. Res. 42, 1995–2004 (2009).
West, J. G., Huang, D. & Sorensen, E. J. Acceptorless dehydrogenation of small molecules through cooperative base metal catalysis. Nat. Commun. 6, 10093 (2015).
Zheng, Y.-W. et al. Photocatalytic hydrogen-evolution cross-couplings: benzene C–H amination and hydroxylation. J. Am. Chem. Soc. 138, 10080–10083 (2016).
Zhang, G. et al. Anti-Markovnikov oxidation of β-alkyl styrenes with H2O as the terminal oxidant. J. Am. Chem. Soc. 138, 12037–12040 (2016).
Niu, L. et al. Photo-induced oxidant-free oxidative C–H/N–H cross-coupling between arenes and azoles. Nat. Commun. 8, 14226 (2017).
Fontecilla-Camps, J. C., Volbeda, A., Cavazza, C. & Nicolet, Y. Structure/function relationships of [NiFe]- and [FeFe]-hydrogenases. Chem. Rev. 107, 4273–4303 (2007).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Pattenden, G. Cobalt-mediated radical reactions in organic synthesis. Chem. Soc. Rev. 17, 361–382 (1988).
Johnston, C. P., Smith, R. T., Allmendinger, S. & MacMillan, D. W. C. Metallaphotoredox-catalysed sp 3–sp 3 cross-coupling of carboxylic acids with alkyl halides. Nature 536, 322–325 (2016).
Bloom, S. et al. Decarboxylative alkylation for site-selective bioconjugation of native proteins via oxidation potentials. Nat. Chem. 10, 205–211 (2018).
Griffin, J. D., Zeller, M. A. & Nicewicz, D. A. Hydrodecarboxylation of carboxylic and malonic acid derivatives via organic photoredox catalysis: substrate scope and mechanistic insight. J. Am. Chem. Soc. 137, 11340–11348 (2015).
Schrauzer, G. N., Sibert, J. W. & Windgassen, R. J. Photochemical and thermal cobalt–carbon bond cleavage in alkylcobalamins and related organometallic compounds. A comparative study. J. Am. Chem. Soc. 90, 6681–6688 (1968).
Shen, R. & Porco, J. A. Jr. Synthesis of enamides related to the salicylate antitumor macrolides using copper-mediated vinylic substitution. Org. Lett. 2, 1333–1336 (2000).
Jiang, L., Job, G. E., Klapars, A. & Buchwald, S. L. Copper-catalyzed coupling of amides and carbamates with vinyl halides. Org. Lett. 5, 3667–3669 (2003).
Grubbs, R. H. Handbook of Metathesis (Wiley, Weinheim, 2003).
Kolb, H. C., VanNieuwenhze, M. S. & Sharpless, K. B. Catalytic asymmetric dihydroxylation. Chem. Rev. 94, 2483–2547 (1994).
Acknowledgements
We thank A. Deege, M. S. Sterling and H. Hinrichs (Max-Planck-Institut für Kohlenforschung) for liquid chromatography analysis, and G. Breitenbruch (Max-Planck-Institut für Kohlenforschung) for HPLC purification. We thank J. Rust and H. Lee (Max-Planck-Institut für Kohlenforschung) for X-ray crystallographic analysis, and M. Blumenthal, D. Kampen, S. Marcus, D. Richter and D. Margold (Max-Planck-Institut für Kohlenforschung) for mass spectrometry. We thank S. Ruthe (Max-Planck-Institut für Kohlenforschung) for gas chromatography analysis. We thank W. S. Ham (Max-Planck-Institut für Kohlenforschung) for help with quantum yield measurements. We thank H. Zhou (Max-Planck-Institut für Kohlenforschung) for help with enantiomertic excess measurements.
Author information
Authors and Affiliations
Contributions
X.S. developed the conceptual approach to the project and optimized the dehydrogenative decarboxyolefination reaction. X.S. and J.C. synthesized the starting materials. X.S. explored the substrate scope and conducted the mechanistic studies. X.S. and T.R. analysed the data and wrote the manuscript. X.S., J.C. and T.R. prepared the Supplementary Information. T.R. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary experimental data, synthetic procedures, chemical compound characterization data and supplementary figures
Crystallographic data
CIF for compound 1; CCDC reference: 1831368
Rights and permissions
About this article
Cite this article
Sun, X., Chen, J. & Ritter, T. Catalytic dehydrogenative decarboxyolefination of carboxylic acids. Nature Chem 10, 1229–1233 (2018). https://doi.org/10.1038/s41557-018-0142-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0142-4
This article is cited by
-
Bismuth radical catalysis in the activation and coupling of redox-active electrophiles
Nature Chemistry (2023)
-
Decarboxylative oxidation-enabled consecutive C-C bond cleavage
Nature Communications (2022)
-
Bioinspired desaturation of alcohols enabled by photoredox proton-coupled electron transfer and cobalt dual catalysis
Nature Communications (2022)
-
Acceptorless dehydrogenative amination of alkenes for the synthesis of N-heterocycles
Science China Chemistry (2022)
-
Silane- and peroxide-free hydrogen atom transfer hydrogenation using ascorbic acid and cobalt-photoredox dual catalysis
Nature Communications (2021)