Abstract
Prostate-specific membrane antigen (PSMA) positron emission tomography (PET) is rapidly being established as arguably the leading contemporary imaging modality in the management of prostate cancer. Outside of its conventional use in the de novo staging of localized disease and detection of biochemical recurrence, additional applications for the use of PSMA PET are emerging. Uptake of PSMA tracers in other genitourinary malignancies, particularly renal cell carcinoma, has led to new fields of investigation. Therapeutic delivery of radiolabelled PSMA small molecules has shown considerable promise in advanced prostate cancer. The ability to use the same molecule for imaging and therapy — theranostics — enables a highly personalized approach. PSMA PET can also have a considerable influence in the selection and guidance of radiotherapy fields for high-risk and recurrent disease. Intriguingly, changes in intensity of PSMA uptake during systemic therapy might provide early response assessment or novel insight into the biological responses of genitourinary malignancies to treatment. An evolving range of radiolabelled PSMA radiopharmaceuticals is emerging in the multiple facets of modern clinical practice.
Key points
Prostate-specific membrane antigen (PSMA) positron emission tomography (PET) has most commonly been used for staging prostate cancer, with most studies in the setting of biochemical recurrence. However, PSMA is not expressed ubiquitously in prostate cancer and PSMA can also be expressed in other solid organ malignancies and benign lesions.
The effect of PSMA PET has been demonstrated in both the definitive and salvage radiotherapy setting through modification of treatment fields.
As PSMA PET has superior accuracy to choline PET–CT, metastasis-directed therapy (including stereotactic ablative body radiotherapy) can be undertaken in the setting of oligometastatic disease.
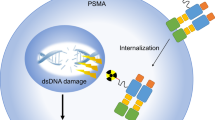
PSMA theranostics (using radionuclides to target PSMA) has been evaluated in advanced disease with promising results in phase II trials.
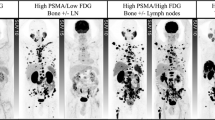
PSMA PET might have a role as an imaging biomarker in assessing response to systemic therapy.
The utility of PSMA PET in other genitourinary malignancies has been studied. It might have a role in metastatic renal cell carcinoma, but there seems to be no role for PSMA PET in urothelial carcinoma.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Silver, D. A., Pellicer, I., Fair, W. R., Heston, W. D. & Cordon-Cardo, C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin. Cancer Res. 3, 81–85 (1997).
Ghosh, A. & Heston, W. D. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J. Cell Biochem. 91, 528–539 (2004).
Lapidus, R. G., Tiffany, C. W., Isaacs, J. T. & Slusher, B. S. Prostate-specific membrane antigen (PSMA) enzyme activity is elevated in prostate cancer cells. Prostate 45, 350–354 (2000).
Ross, J. S. et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin. Cancer Res. 9, 6357–6362 (2003).
Weissleder, R. & Pittet, M. J. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
Fletcher, J. W. et al. Recommendations on the use of 18F-FDG PET in oncology. J. Nucl. Med. 49, 480–508 (2008).
Powles, T., Murray, I., Brock, C., Oliver, T. & Avril, N. Molecular positron emission tomography and PET/CT imaging in urological malignancies. Eur. Urol. 51, 1511–1521 (2007).
Hillner, B. E. et al. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry. J. Clin. Oncol. 26, 2155–2161 (2008).
Ramdave, S. et al. Clinical role of F-18 fluorodeoxyglucose positron emission tomography for detection and management of renal cell carcinoma. J. Urol. 166, 825–830 (2001).
Albers, P. et al. Testicular cancer (EAU guidelines). Uroweb https://uroweb.org/guideline/testicular-cancer/ (2017).
Reske, S. N. et al. Imaging prostate cancer with 11C-choline PET/CT. J. Nucl. Med. 47, 1249–1254 (2006).
Afshar-Oromieh, A. et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 41, 11–20 (2014).
Afshar-Oromieh, A., Haberkorn, U., Eder, M., Eisenhut, M. & Zechmann, C. M. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur. J. Nucl. Med. Mol. Imaging 39, 1085–1086 (2012).
Eder, M. et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug. Chem. 23, 688–697 (2012).
Cornford, P. et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 71, 630–642 (2017).
NCCN. NCCN Guidelines in Oncology. Prostate Cancer. Version 3.2016. NCCN https://www.nccn.org/professionals/physician_gls/ (2016).
Szabo, Z. et al. Initial evaluation of [(18)F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol. Imaging Biol. 17, 565–574 (2015).
Giesel, F. L. et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 44, 678–688 (2017).
Zschaeck, S. et al. PSMA-PET based radiotherapy: a review of initial experiences, survey on current practice and future perspectives. Radiat. Oncol. 13, 90 (2018).
Ong, W. M., Zargar-Shoshtari, K., Siva, S. & Zargar, H. Prostate specific membrane antigen: the role in salvage lymph node dissection and radio-ligand therapy. Minerva Urol. Nefrol. 70, 450–461 (2018).
Van Leeuwen, P. J. et al. 68Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int. 117, 732–739 (2016).
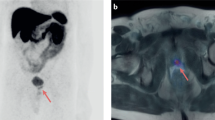
Rauscher, I., Horn, T., Eiber, M., Gschwend, J. E. & Maurer, T. Novel technology of molecular radio-guidance for lymph node dissection in recurrent prostate cancer by PSMA-ligands. World J. Urol. 36, 603–608 (2018).
Maurer, T. et al. (99m)Technetium-based Prostate-specific membrane antigen-radioguided surgery in recurrent prostate cancer. Eur. Urol. 75, 659–666 (2019).
Meershoek, P. et al. Robot-assisted laparoscopic surgery using DROP-IN radioguidance: first-in-human translation. Eur. J. Nucl. Med. Mol. Imaging 46, 49–53 (2019).
Scher, H. I. et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J. Clin. Oncol. 26, 1148–1159 (2008).
Clarebrough, E., Duncan, C., Christidis, D., Lavoipierre, A. & Lawrentschuk, N. PSMA-PET guided hook-wire localization of nodal metastases in prostate cancer: a targeted approach. World J. Urol. 37, 1251–1254 (2019).
Israeli, R. S., Powell, C. T., Corr, J. G., Fair, W. R. & Heston, W. D. Expression of the prostate-specific membrane antigen. Cancer Res. 54, 1807–1811 (1994).
Demirci, E. et al. Normal distribution pattern and physiological variants of 68Ga-PSMA-11 PET/CT imaging. Nucl. Med. Commun. 37, 1169–1179 (2016).
Rischpler, C. et al. (68)Ga-PSMA-HBED-CC Uptake in cervical, celiac, and sacral ganglia as an important pitfall in prostate cancer PET imaging. J. Nucl. Med. 59, 1406–1411 (2018).
Beheshti, M., Rezaee, A. & Langsteger, W. 68Ga-PSMA-HBED uptake on cervicothoracic (stellate) ganglia, a common pitfall on PET/CT. Clin. Nucl. Med. 42, 195–196 (2017).
Schwarzenboeck, S. M. et al. PSMA ligands for PET imaging of prostate cancer. J. Nucl. Med. 58, 1545–1552 (2017).
Hofman, M. S., Hicks, R. J., Maurer, T. & Eiber, M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. RadioGraphics 38, 200–217 (2018).
Salas Fragomeni, R. A. et al. Imaging of nonprostate cancers using PSMA-targeted radiotracers: rationale, current state of the field, and a call to arms. J. Nucl. Med. 59, 871–877 (2018).
Baccala, A., Sercia, L., Li, J., Heston, W. & Zhou, M. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology 70, 385–390 (2007).
Haffner, M. C. et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum. Pathol. 40, 1754–1761 (2009).
Maurer, T. et al. Diagnostic efficacy of (68)gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J. Urol. 195, 1436–1443 (2016).
Broos, W. A. M., Kocken, M., van der Zant, F. M., Knol, R. J. J. & Wondergem, M. Metastasized 18F-DCFPyL-negative prostatic adenocarcinoma without neuroendocrine differentiation. Clin. Nucl. Med. 43, 120–122 (2018).
Usmani, S. et al. Molecular imaging in neuroendocrine differentiation of prostate cancer: 68Ga-PSMA versus 68Ga-DOTA NOC PET-CT. Clin. Nucl. Med. 42, 410–413 (2017).
Thang, S. P. et al. Poor outcomes for patients with metastatic castration-resistant prostate cancer with low prostate-specific membrane antigen (PSMA) expression deemed ineligible for (177)Lu-labelled PSMA radioligand therapy. Eur. Urol. Oncol. 2, 670–676 (2018).
Demirci, E. et al. (68)Ga-PSMA PET/CT imaging of metastatic clear cell renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 41, 1461–1462 (2014).
Campbell, S. P. et al. Low levels of PSMA expression limit the utility of 18 F-DCFPyL PET/CT for imaging urothelial carcinoma. Ann. Nucl. Med. 32, 69–74 (2018).
Koerber, S. A. et al. (68)Ga-PSMA-11 PET/CT in Primary and recurrent prostate carcinoma: implications for radiotherapeutic management in 121 patients. J. Nucl. Med. 60, 234–240 (2019).
Emmett, L. et al. Rapid modulation of psma expression by androgen deprivation: serial (68)Ga-PSMA-11 PET in men with hormone-sensitive and castrate-resistant prostate cancer commencing androgen blockade. J. Nucl. Med. 60, 950–954 (2019).
Prasad, V. et al. Biodistribution of [68Ga] PSMA-HBED-CC in patients with prostate cancer: characterization of uptake in normal organs and tumour lesions. Mol. Imaging Biol. 18, 428–436 (2016).
Sasikumar, A., Joy, A., Nanabala, R., Unni, M. & Tk, P. Complimentary pattern of uptake in 18F-FDG PET/CT and 68Ga-prostate-specific membrane antigen PET/CT in a case of metastatic clear cell renal carcinoma. Clin. Nucl. Med. 41, e517–e519 (2016).
Siva, S. et al. Utility of 68 Ga prostate specific membrane antigen - positron emission tomography in diagnosis and response assessment of recurrent renal cell carcinoma. J. Med. Imaging Radiat. Oncol. 61, 372–378 (2017).
Kabasakal, E. D. L. & Kanmaz, M. H. B. 68Ga-PSMA PET/CT imaging of metastatic clear cell renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 41, 1461–1462 (2014).
Rowe, S. P. et al. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann. Nucl. Med. 29, 877–882 (2015).
Nadebaum, D. P., Hofman, M. S., Mitchell, C. A., Siva, S. & Hicks, R. J. Oligometastatic renal cell carcinoma with sarcomatoid differentiation demonstrating variable imaging phenotypes on (68)ga-psma and (18)f-fdg pet/ct: a case report and review of the literature. Clin. Genitourin. Cancer https://doi.org/10.1016/j.clgc.2017.08.009 (2017).
Rhee, H. et al. Pilot study: use of gallium-68 PSMA PET for detection of metastatic lesions in patients with renal tumour. EJNMMI Res. 6, 76 (2016).
Yin, Y. et al. Inconsistent detection of sites of metastatic non-clear cell renal cell carcinoma with PSMA-targeted [(18)F]DCFPyL PET/CT. Mol. Imaging Biol. 21, 567–573 (2019).
Spatz, S. et al. Comprehensive evaluation of prostate specific membrane antigen expression in the vasculature of renal tumors: implications for imaging studies and prognostic role. J. Urol. 199, 370–377 (2018).
Mhawech-Fauceglia, P. et al. Prostate-specific membrane antigen (PSMA) protein expression in normal and neoplastic tissues and its sensitivity and specificity in prostate adenocarcinoma: an immunohistochemical study using mutiple tumour tissue microarray technique. Histopathology 50, 472–483 (2007).
Gala, J.-L. et al. Expression of prostate-specific membrane antigen in transitional cell carcinoma of the bladder: prognostic value? Clin. Cancer Res. 6, 4049–4054 (2000).
Tagawa, S. T. et al. Phase II study of lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 19, 5182–5191 (2013).
Vallabhajosula, S. et al. Radioimmunotherapy of prostate cancer using 90Y- and 177Lu-labeled J591 monoclonal antibodies: effect of multiple treatments on myelotoxicity. Clin. Cancer Res. 11, 7195s–7200s (2005).
Bander, N. H. et al. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J. Clin. Oncol. 23, 4591–4601 (2005).
Bander, N. H. et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J. Urol. 170, 1717–1721 (2003).
Hofman, M. S. et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 19, 825–833 (2018).
Ahmadzadehfar, H. et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 5, 114 (2015).
Rahbar, K. et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J. Nucl. Med. 58, 85–90 (2017).
Kratochwil, C. et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 57, 1170–1176 (2016).
Yadav, M. P. et al. (177)Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur. J. Nucl. Med. Mol. Imaging 44, 81–91 (2017).
Heck, M. M. et al. Systemic radioligand therapy with (177)Lu Labeled prostate specific membrane antigen ligand for imaging and therapy in patients with metastatic castration resistant prostate cancer. J. Urol. 196, 382–391 (2016).
Kulkarni, H. R. et al. PSMA-Based radioligand therapy for metastatic castration-resistant prostate cancer: the bad berka experience since 2013. J. Nucl. Med. 57, 97S–104S (2016).
Baum, R. P. et al. 177Lu-Labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J. Nucl. Med. 57, 1006–1013 (2016).
Fendler, W. P. et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 8, 3581–3590 (2017).
Baum, R. P. & Wahl, R. L. Third theranostics world congress on gallium-68 and PRRT: abstracts. J. Nucl. Med. 56, 2A–30 (2015).
Zechmann, C. M. et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 41, 1280–1292 (2014).
Kratochwil, C. et al. [(1)(7)(7)Lu]Lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 42, 987–988 (2015).
Heck, M. M. et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with (177)Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur. Urol. 75, 920–926 (2019).
Fendler, W. P. et al. Prostate-specific membrane antigen ligand positron-emission tomography in men with nonmetastatic castration-resistant prostate cancer. Clin. Cancer Res. 25, 7448–7454 (2019).
US National Library of Medicine. ClinicalTrials.Gov https://ClinicalTrials.gov/show/NCT03511664 (2019).
Hekman, M. C. H. et al. Positron emission tomography/computed tomography with (89)Zr-girentuximab can aid in diagnostic dilemmas of clear cell renal cell carcinoma suspicion. Eur. Urol. 74, 257–260 (2018).
Calais, J. et al. Potential impact of (68)Ga-PSMA-11 PET/CT on the planning of definitive radiation therapy for prostate cancer. J. Nucl. Med. 59, 1714–1721 (2018).
Michalski, J. M. et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 76, 361–368 (2010).
Zschaeck, S. et al. Intermediate-term outcome after PSMA-PET guided high-dose radiotherapy of recurrent high-risk prostate cancer patients. Radiat. Oncol. 12, 140 (2017).
Habl, G. et al. (68) Ga-PSMA-PET for radiation treatment planning in prostate cancer recurrences after surgery: individualized medicine or new standard in salvage treatment. Prostate 77, 920–927 (2017).
Schiller, K. et al. Patterns of failure after radical prostatectomy in prostate cancer - implications for radiation therapy planning after (68)Ga-PSMA-PET imaging. Eur. J. Nucl. Med. Mol. Imaging 44, 1656–1662 (2017).
Frenzel, T. et al. The impact of [(68)Ga]PSMA I&T PET/CT on radiotherapy planning in patients with prostate cancer. Strahlenther. Onkol. 194, 646–654 (2018).
Calais, J. et al. (68)Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a psa level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J. Nucl. Med. 59, 230–237 (2018).
Tosoian, J. J. et al. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nat. Rev. Urol. 14, 15–25 (2017).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
Palma, D. A. et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 393, 2051–2058 (2019).
Ost, P. et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur. Urol. 67, 852–863 (2015).
Fossati, N. et al. Identifying the optimal candidate for salvage lymph node dissection for nodal recurrence of prostate cancer: results from a large, multi-institutional analysis. Eur. Urol. 75, 176–183 (2019).
Ost, P. et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J. Clin. Oncol. 36, 446–453 (2018).
Siva, S. et al. Stereotactic abative body radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur. Urol. 74, 455–462 (2018).
Pfister, D. et al. Detection of recurrent prostate cancer lesions before salvage lymphadenectomy is more accurate with (68)Ga-PSMA-HBED-CC than with (18)F-fluoroethylcholine PET/CT. Eur. J. Nucl. Med. Mol. Imaging 43, 1410–1417 (2016).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Even-Sapir, E. Imaging of malignant bone involvement by morphologic, scintigraphic, and hybrid modalities. J. Nucl. Med. 46, 1356–1367 (2005).
Udovicich, C. et al. 68Ga-prostate-specific membrane antigen-positron emission tomography/computed tomography in advanced prostate cancer: current state and future trends. Prostate Int. 5, 125–129 (2017).
Miyahira, A. K. et al. Tumor cell heterogeneity and resistance; report from the 2018 coffey-holden prostate cancer academy meeting. Prostate 79, 244–258 (2019).
Davies, A. H., Beltran, H. & Zoubeidi, A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat. Rev. Urol. 15, 271–286 (2018).
Wang, H. T. et al. Neuroendocrine prostate cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J. Clin. Oncol. 32, 3383–3390 (2014).
Akamatsu, S., Inoue, T., Ogawa, O. & Gleave, M. E. Clinical and molecular features of treatment-related neuroendocrine prostate cancer. Int. J. Urol. 25, 345–351 (2018).
Tosoian, J. J. et al. Correlation of PSMA-Targeted (18)F-DCFPyL PET/CT findings with immunohistochemical and genomic data in a patient with metastatic neuroendocrine prostate cancer. Clin. Genitourin. Cancer 15, e65–e68 (2017).
Bronsert, P., Reichel, K. & Ruf, J. Loss of PSMA expression in non-neuroendocrine dedifferentiated acinar prostate cancer. Clin. Nucl. Med. 43, 526–528 (2018).
Meller, B. et al. Alterations in androgen deprivation enhanced prostate-specific membrane antigen (PSMA) expression in prostate cancer cells as a target for diagnostics and therapy. EJNMMI Res. 5, 66 (2015).
Anton, A. et al. 223PUsing PSMA PET/CT to assess response in metastatic prostate cancer (mPC) patients (pts) receiving upfront chemohormonal therapy. Ann. Oncol. 29, mdy434.011–mdy434.011 (2018).
Seitz, A. K. et al. Preliminary results on response assessment using (68)Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 45, 602–612 (2018).
Steuber, T. et al. Standard of care versus metastases-directed therapy for pet-detected nodal oligorecurrent prostate cancer following multimodality treatment: a multi-institutional case-control study. Eur. Urol. Focus 5, 1007–1013 (2018).
Lohaus, F. et al. Can local ablative radiotherapy revert castration-resistant prostate cancer to an earlier stage of disease? Eur. Urol. 75, 548–551 (2019).
Gomez, D. R. et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J. Clin. Oncol. 37, 1558–1565 (2019).
US National Library of Medicine. ClinicalTrials.Gov https://clinicaltrials.gov/ct2/show/NCT02685397 (2018).
Kranzbuhler, B. et al. Pharmacological upregulation of prostate-specific membrane antigen (PSMA) expression in prostate cancer cells. Prostate 78, 758–765 (2018).
Afshar-Oromieh, A. et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 45, 2045–2054 (2018).
Tsui, P., Rubenstein, M. & Guinan, P. Correlation between PSMA and VEGF expression as markers for LNCaP tumor angiogenesis. J. Biomed. Biotechnol. 2005, 287–290 (2005).
Acknowledgements
S.S. is supported through a National Health and Medical Research Council Fellowship APP1122347 and Peter Mac Discovery Partner Fellowship.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussion of the article content, wrote the manuscript, and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Urology thanks T. Maurer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Siva, S., Udovicich, C., Tran, B. et al. Expanding the role of small-molecule PSMA ligands beyond PET staging of prostate cancer. Nat Rev Urol 17, 107–118 (2020). https://doi.org/10.1038/s41585-019-0272-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-019-0272-5