Abstract
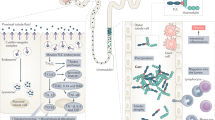
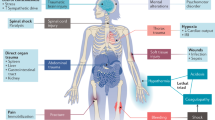
Intravascular haemolysis is a fundamental feature of chronic hereditary and acquired haemolytic anaemias, including those associated with haemoglobinopathies, complement disorders and infectious diseases such as malaria. Destabilization of red blood cells (RBCs) within the vasculature results in systemic inflammation, vasomotor dysfunction, thrombophilia and proliferative vasculopathy. The haemoprotein scavengers haptoglobin and haemopexin act to limit circulating levels of free haemoglobin, haem and iron — potentially toxic species that are released from injured RBCs. However, these adaptive defence systems can fail owing to ongoing intravascular disintegration of RBCs. Induction of the haem-degrading enzyme haem oxygenase 1 (HO1) — and potentially HO2 — represents a response to, and endogenous defence against, large amounts of cellular haem; however, this system can also become saturated. A frequent adverse consequence of massive and/or chronic haemolysis is kidney injury, which contributes to the morbidity and mortality of chronic haemolytic diseases. Intravascular destruction of RBCs and the resulting accumulation of haemoproteins can induce kidney injury via a number of mechanisms, including oxidative stress and cytotoxicity pathways, through the formation of intratubular casts and through direct as well as indirect proinflammatory effects, the latter via the activation of neutrophils and monocytes. Understanding of the detailed pathophysiology of haemolysis-induced kidney injury offers opportunities for the design and implementation of new therapeutic strategies to counteract the unfavourable and potentially fatal effects of haemolysis on the kidney.
Key points
-
Several human haemolytic conditions result in the presence of large amounts of haemoglobin and haem in the circulation, which overwhelms endogenous scavengers such as haptoglobin and haemopexin.
-
Free haemoproteins present in plasma are filtered by the kidney, exposing the kidney to the injurious effects of haem and iron.
-
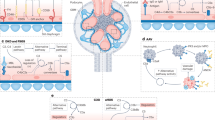
The presence of copious quantities of haem in the kidney necessitates clearance of haem; induction of haem oxygenase 1 and ferritin in the kidney protects against haem-induced oxidative stress.
-
However, these mechanisms are not sufficient to avoid pathological outcomes instigated by cell-free haemoglobin, haem and iron during haemolytic conditions such as oxidative stress, nitric oxide depletion, inflammation and cell death.
-
Haemoprotein-induced acute kidney injury is a multifactorial process, involving reactive oxygen species, labile iron and inflammation.
-
Two main approaches exist for the treatment of haemolytic anaemias: treating the underlying disease to prevent the disintegration of red blood cells and mitigating the damage induced by released haemoproteins.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bancroft, F. W. Anuria following transfusion: effect of decapsulation of both kidneys. Ann. Surg. 81, 733–738 (1925).
Bywaters, E. G. & Beall, D. Crush injuries with impairment of renal function. Br. Med. J. 1, 427–432 (1941).
Muckenthaler, M. U., Rivella, S., Hentze, M. W. & Galy, B. A red carpet for iron metabolism. Cell 168, 344–361 (2017).
Rother, R. P., Bell, L., Hillmen, P. & Gladwin, M. T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 293, 1653–1662 (2005).
Jakeman, G. N., Saul, A., Hogarth, W. L. & Collins, W. E. Anaemia of acute malaria infections in non-immune patients primarily results from destruction of uninfected erythrocytes. Parasitology 119 (Pt 2), 127–133 (1999).
White, N. J. Anaemia and malaria. Malar. J. 17, 371 (2018).
Ackers, G. K. & Halvorson, H. R. The linkage between oxygenation and subunit dissociation in human hemoglobin. Proc. Natl Acad. Sci. USA 71, 4312–4316 (1974).
Matheson, B., Razynska, A., Kwansa, H. & Bucci, E. Appearance of dissociable and cross-linked hemoglobins in the renal hilar lymph. J. Lab. Clin. Med. 135, 459–464 (2000).
Nakai, K. et al. Permeability characteristics of hemoglobin derivatives across cultured endothelial cell monolayers. J. Lab. Clin. Med. 132, 313–319 (1998).
Butt, O. I., Buehler, P. W. & D’Agnillo, F. Differential induction of renal heme oxygenase and ferritin in ascorbate and nonascorbate producing species transfused with modified cell-free hemoglobin. Antioxid. Redox Signal. 12, 199–208 (2010).
Donadee, C. et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation 124, 465–476 (2011).
Doherty, D. H. et al. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nat. Biotechnol. 16, 672–676 (1998).
Reiter, C. D. et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 8, 1383–1389 (2002).
Yeo, T. W. et al. Greater endothelial activation, Weibel–Palade body release and host inflammatory response to Plasmodium vivax, compared with Plasmodium falciparum: a prospective study in Papua, Indonesia. J. Infect. Dis. 202, 109–112 (2010).
Janka, J. J. et al. Increased pulmonary pressures and myocardial wall stress in children with severe malaria. J. Infect. Dis. 202, 791–800 (2010).
Hill, A. et al. Effect of eculizumab on haemolysis-associated nitric oxide depletion, dyspnoea, and measures of pulmonary hypertension in patients with paroxysmal nocturnal haemoglobinuria. Br. J. Haematol. 149, 414–425 (2010).
Olson, J. S. et al. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic. Biol. Med. 36, 685–697 (2004).
Minneci, P. C. et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J. Clin. Invest. 115, 3409–3417 (2005).
Wesson, D. E. The initiation and progression of sickle cell nephropathy. Kidney Int. 61, 2277–2286 (2002).
Hostetter, T. H. Progression of renal disease and renal hypertrophy. Annu. Rev. Physiol. 57, 263–278 (1995).
Hostetter, T. H. Hyperfiltration and glomerulosclerosis. Semin. Nephrol. 23, 194–199 (2003).
Nath, K. A. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am. J. Kidney Dis. 20, 1–17 (1992).
Kriz, W. & LeHir, M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 67, 404–419 (2005).
Baek, J. H. et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J. Clin. Invest. 122, 1444–1458 (2012).
Morrow, J. D. et al. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl Acad. Sci. USA 87, 9383–9387 (1990).
Takahashi, K. et al. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J. Clin. Invest. 90, 136–141 (1992).
Nath, K. A., Balla, J., Croatt, A. J. & Vercellotti, G. M. Heme protein-mediated renal injury: a protective role for 21-aminosteroids in vitro and in vivo. Kidney Int. 47, 592–602 (1995).
Balla, J. et al. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc. Natl Acad. Sci. USA 90, 9285–9289 (1993).
Boutaud, O. et al. Acetaminophen inhibits hemoprotein-catalyzed lipid peroxidation and attenuates rhabdomyolysis-induced renal failure. Proc. Natl Acad. Sci. USA 107, 2699–2704 (2010).
Lipiski, M. et al. Human Hp1-1 and Hp2-2 phenotype-specific haptoglobin therapeutics are both effective in vitro and in guinea pigs to attenuate hemoglobin toxicity. Antioxid. Redox Signal. 19, 1619–1633 (2013).
Bunn, H. F. & Jandl, J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J. Biol. Chem. 243, 465–475 (1968).
Gattoni, M., Boffi, A., Sarti, P. & Chiancone, E. Stability of the heme-globin linkage in dimers and isolated chains of human hemoglobin. A study of the heme transfer reaction from the immobilized proteins to albumin. J. Biol. Chem. 271, 10130–10136 (1996).
Mollan, T. L. et al. Redox properties of human hemoglobin in complex with fractionated dimeric and polymeric human haptoglobin. Free Radic. Biol. Med. 69, 265–277 (2014).
Natanson, C., Kern, S. J., Lurie, P., Banks, S. M. & Wolfe, S. M. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA 299, 2304–2312 (2008).
Vincent, S. H. Oxidative effects of heme and porphyrins on proteins and lipids. Semin. Hematol. 26, 105–113 (1989).
Balla, G., Jacob, H. S., Eaton, J. W., Belcher, J. D. & Vercellotti, G. M. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler. Thromb. 11, 1700–1711 (1991).
Vercellotti, G. M. et al. Heme and the vasculature: an oxidative hazard that induces antioxidant defenses in the endothelium. Artif. Cells Blood Substit. Immobil. Biotechnol. 22, 207–213 (1994).
Jeney, V. et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood 100, 879–887 (2002).
Balla, G., Vercellotti, G. M., Muller-Eberhard, U., Eaton, J. & Jacob, H. S. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab. Invest. 64, 648–655 (1991).
Kumar, S. & Bandyopadhyay, U. Free heme toxicity and its detoxification systems in human. Toxicol. Lett. 157, 175–188 (2005).
Ryter, S. W. & Tyrrell, R. M. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic. Biol. Med. 28, 289–309 (2000).
Tolosano, E., Fagoonee, S., Morello, N., Vinchi, F. & Fiorito, V. Heme scavenging and the other facets of hemopexin. Antiox. Redox Signal. 12, 305–320 (2010).
Larsen, R. et al. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl Med. 2, 51ra71 (2010).
Hod, E. A. et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood 115, 4284–4292 (2010).
Ponka, P. Cell biology of heme. Am. J. Med. Sci. 318, 241–256 (1999).
Wagener, F. A. et al. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol. Rev. 55, 551–571 (2003).
Reeder, B. J. The redox activity of hemoglobins: from physiologic functions to pathologic mechanisms. Antioxid. Redox Signal. 13, 1087–1123 (2010).
Hamza, I. & Dailey, H. A. One ring to rule them all: trafficking of heme and heme synthesis intermediates in the metazoans. Biochim. Biophys. Acta 1823, 1617–1632 (2012).
Soares, M. P. & Weiss, G. The Iron age of host-microbe interactions. EMBO Rep. 16, 1482–1500 (2015).
Chance, B. The reactivity of haemoproteins and cytochromes. Biochem. J. 103, 1–18 (1967).
Mense, S. M. & Zhang, L. Heme: a versatile signaling molecule controlling the activities of diverse regulators ranging from transcription factors to MAP kinases. Cell Res. 16, 681–692 (2006).
Boretti, F. S. et al. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J. Clin. Invest. 119, 2271–2280 (2009).
Gladwin, M. T., Kanias, T. & Kim-Shapiro, D. B. Hemolysis and cell-free hemoglobin drive an intrinsic mechanism for human disease. J. Clin. Invest. 122, 1205–1208 (2012).
Kristiansen, M. et al. Identification of the haemoglobin scavenger receptor. Nature 409, 198–201 (2001).
Schaer, C. A., Vallelian, F., Imhof, A., Schoedon, G. & Schaer, D. J. CD163-expressing monocytes constitute an endotoxin-sensitive Hb clearance compartment within the vascular system. J. Leukoc. Biol. 82, 106–110 (2007).
Schaer, D. J. et al. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood 107, 373–380 (2006).
Schaer, C. A., Schoedon, G., Imhof, A., Kurrer, M. O. & Schaer, D. J. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ. Res. 99, 943–950 (2006).
Kassa, T., Jana, S., Meng, F. & Alayash, A. I. Differential heme release from various hemoglobin redox states and the upregulation of cellular heme oxygenase-1. FEBS Open Bio. 6, 876–884 (2016).
Ascenzi, P. et al. Hemoglobin and heme scavenging. IUBMB Life 57, 749–759 (2005).
Muller-Eberhard, U. & Cleve, H. Immunoelectrophoretic studies of the beta1-haem-binding globulin (haemopexin) in hereditary haemolytic disorders. Nature 197, 602–603 (1963).
Muller-Eberhard, U. Hemopexin. N. Engl. J. Med. 283, 1090–1094 (1970).
Tolosano, E. & Altruda, F. Hemopexin: structure, function, and regulation. DNA Cell Biol. 21, 297–306 (2002).
Paoli, M. et al. Crystal structure of hemopexin reveals a novel high-affinity heme site formed between two β-propeller domains. Nat. Struct. Biol. 6, 926–931 (1999).
Gutteridge, J. M. & Smith, A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem. J. 256, 861–865 (1988).
Vercellotti, G. M. et al. Hepatic overexpression of hemopexin inhibits inflammation and vascular stasis in murine models of sickle cell disease. Mol. Med. 22, 437–451 (2016).
Potter, D. et al. In vivo fate of hemopexin and heme-hemopexin complexes in the rat. Arch. Biochem. Biophys. 300, 98–104 (1993).
Hvidberg, V. et al. Identification of the receptor scavenging hemopexin-heme complexes. Blood 106, 2572–2579 (2005).
Smith, A. & Morgan, W. T. Haem transport to the liver by haemopexin. Receptor-mediated uptake with recycling of the protein. Biochem. J. 182, 47–54 (1979).
Vinchi, F., Gastaldi, S., Silengo, L., Altruda, F. & Tolosano, E. Hemopexin prevents endothelial damage and liver congestion in a mouse model of heme overload. Am. J. Pathol. 173, 289–299 (2008).
Tolosano, E. et al. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood 94, 3906–3914 (1999).
Vinchi, F. et al. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation 127, 1317–1329 (2013).
Muller-Eberhard, U., Javid, J., Liem, H. H., Hanstein, A. & Hanna, M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood 32, 811–815 (1968).
Kutty, R. K. & Maines, M. D. Purification and characterization of biliverdin reductase from rat liver. J. Biol. Chem. 256, 3956–3962 (1981).
Maines, M. D. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 37, 517–554 (1997).
Ryter, S. W., Alam, J. & Choi, A. M. K. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 86, 583–650 (2006).
Nath, K. A. et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J. Clin. Invest. 90, 267–270 (1992).
Kovtunovych, G., Eckhaus, M. A., Ghosh, M. C., Ollivierre-Wilson, H. & Rouault, T. A. Dysfunction of the heme recycling system in heme oxygenase 1–deficient mice: effects on macrophage viability and tissue iron distribution. Blood 116, 6054–6062 (2010).
Yachie, A. et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 103, 129–135 (1999).
Koizumi, S. Human heme oxygenase-1 deficiency: a lesson on serendipity in the discovery of the novel disease. Pediatr. Int. 49, 125–132 (2007).
Tzima, S., Victoratos, P., Kranidioti, K., Alexiou, M. & Kollias, G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN- β production. J. Exp. Med. 206, 1167–1179 (2009).
Wagener, F. A. et al. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood 98, 1802–1811 (2001).
Kapturczak, M. H. et al. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 165, 1045–1053 (2004).
Doré, S. et al. Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury. Proc. Natl Acad. Sci. USA 96, 2445–2450 (1999).
Soares, M. P. et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 172, 3553–3563 (2004).
Kawamura, K. et al. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler. Thromb. Vasc. Biol. 25, 155–160 (2005).
Ndisang, J. F., Wu, L., Zhao, W. & Wang, R. Induction of heme oxygenase-1 and stimulation of cGMP production by hemin in aortic tissues from hypertensive rats. Blood 101, 3893–3900 (2003).
Beckman, J. D. et al. Inhaled carbon monoxide reduces leukocytosis in a murine model of sickle cell disease. Am. J. Physiol. Heart Circ. Physiol. 297, H1243–H1253 (2009).
Belcher, J. D. et al. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J. Clin. Invest. 116, 808–816 (2006).
Belcher, J. D. et al. Heme oxygenase-1 gene delivery by Sleeping Beauty inhibits vascular stasis in a murine model of sickle cell disease. J. Mol. Med. 88, 665–675 (2010).
Nath, K. A. et al. Age sensitizes the kidney to heme protein-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 304, F317–F325 (2013).
Balla, G. et al. Ferritin: a cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 267, 18148–18153 (1992).
Berberat, P. O. et al. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 17, 1724–1726 (2003).
Cozzi, A. et al. Analysis of the biologic functions of H- and L-ferritins in HeLa cells by transfection with siRNAs and cDNAs: evidence for a proliferative role of L-ferritin. Blood 103, 2377–2383 (2004).
Pham, C. G. et al. Ferritin heavy chain upregulation by NF-κB inhibits TNFα-induced apoptosis by suppressing reactive oxygen species. Cell 119, 529–542 (2004).
Gozzelino, R. & Soares, M. P. Coupling heme and iron metabolism via ferritin H chain. Antiox. Redox Signal. 20, 1754–1769 (2013).
Balla, J. et al. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol. Nutr. Food Res. 49, 1030–1043 (2005).
Robinson, S. R., Dang, T. N., Dringen, R. & Bishop, G. M. Hemin toxicity: a preventable source of brain damage following hemorrhagic stroke. Redox Report 14, 228–235 (2009).
Roche, M., Rondeau, P., Singh, N. R., Tarnus, E. & Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 582, 1783–1787 (2008).
Belayev, L. et al. Experimental intracerebral hematoma in the rat: characterization by sequential magnetic resonance imaging, behavior, and histopathology. Effect of albumin therapy. Brain Res. 1157, 146–155 (2007).
Wang, P., Wu, J., Gao, Z. & Li, H. Tyrosine residues of bovine serum albumin play an important role in protecting SH-SY5Y cells against heme/H2O2/NO2 −-induced damage. Mol. Cell Biochem. 454, 57–66 (2019).
Monzani, E. et al. Enzymatic properties of human hemalbumin. Biochim. Biophys. Acta 1547, 302–312 (2001).
Huang, Y., Shuai, Y., Li, H. & Gao, Z. Tyrosine residues play an important role in heme detoxification by serum albumin. Biochim. Biophys. Acta 1840, 970–976 (2014).
Zunszain, P. A., Ghuman, J., Komatsu, T., Tsuchida, E. & Curry, S. Crystal structural analysis of human serum albumin complexed with hemin and fatty acid. BMC Struct. Biol. 3, 6 (2003).
Wardell, M. et al. The atomic structure of human methemalbumin at 1.9 Å. Biochem. Biophys. Res. Commun. 291, 813–819 (2002).
Larsen, R., Gouveia, Z., Soares, M. P. & Gozzelino, R. Heme cytotoxicity and the pathogenesis of immune-mediated inflammatory diseases. Front. Pharmacol. 3, 77 (2012).
Balla, J. et al. Ferriporphyrins and endothelium: a 2-edged sword-promotion of oxidation and induction of cytoprotectants. Blood 95, 3442–3450 (2000).
Miller, Y. I. & Shaklai, N. Kinetics of hemin distribution in plasma reveals its role in lipoprotein oxidation. Biochim. Biophys. Acta 1454, 153–164 (1999).
Eskew, J. D., Vanacore, R. M., Sung, L., Morales, P. J. & Smith, A. Cellular protection mechanisms against extracellular heme heme-hemopexin, but not free heme, activates the N-terminal c-Jun kinase. J. Biol. Chem. 274, 638–648 (1999).
Chen, G. et al. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood 123, 3818–3827 (2014).
Belcher, J. D. et al. Heme triggers TLR4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood 123, 377–390 (2014).
Vinchi, F. et al. Hemopexin therapy reverts heme-induced proinflammatory phenotypic switching of macrophages in a mouse model of sickle cell disease. Blood 127, 473–486 (2016).
Dutra, F. F. et al. Hemolysis-induced lethality involves inflammasome activation by heme. Proc. Natl Aacad. Sci. USA 111, E4110–E4118 (2014).
Li, Q. et al. Heme induces IL-1β secretion through activating NLRP3 in kidney inflammation. Cell Biochem. Biophys. 69, 495–502 (2014).
Fortes, G. B. et al. Heme induces programmed necrosis on macrophages through autocrine TNF and ROS production. Blood 119, 2368–2375 (2012).
Figueiredo, R. T. et al. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 282, 20221–20229 (2007).
Van Avondt, K. et al. Free iron in sera of patients with sickle cell disease contributes to the release of neutrophil extracellular traps. Blood 128, 161 (2016).
Silvestre-Roig, C. et al. Externalized histone H4 orchestrates chronic inflammation by inducing lytic cell death. Nature 569, 236–240 (2019).
Van Avondt, K. & Hartl, D. Mechanisms and disease relevance of neutrophil extracellular trap formation. Eur. J. Clin. Invest. 48, e12919 (2018).
Van Avondt, K., Maegdefessel, L. & Soehnlein, O. Therapeutic targeting of neutrophil extracellular traps in atherogenic inflammation. Thromb. Haemost. 119, 542–552 (2019).
Yang, H. et al. Globin attenuates the innate immune response to endotoxin. Shock 17, 485–490 (2002).
Fernandez, P. L. et al. Heme amplifies the innate immune response to microbial molecules through spleen tyrosine kinase (Syk)-dependent reactive oxygen species generation. J. Biol. Chem. 285, 32844–32851 (2010).
Lin, T. et al. Synergistic inflammation is induced by blood degradation products with microbial Toll-like receptor agonists and is blocked by hemopexin. J. Infect. Dis. 202, 624–632 (2010).
Lin, T. et al. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J. Immunol. 189, 2017–2022 (2012).
Wagener, F. A., Feldman, E., de Witte, T. & Abraham, N. G. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1, and E selectin in vascular endothelial cells. Proc. Soc. Exp. Biol. Med. 216, 456–463 (1997).
Frimat, M. et al. Complement activation by heme as a secondary hit for atypical hemolytic uremic syndrome. Blood 122, 282–292 (2013).
Ricklin, D., Hajishengallis, G., Yang, K. & Lambris, J. D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 11, 785–797 (2010).
Ricklin, D., Mastellos, D. C., Reis, E. S. & Lambris, J. D. The renaissance of complement therapeutics. Nat. Rev. Nephrol. 14, 26–47 (2018).
Kolev, M., Le Friec, G. & Kemper, C. Complement-tapping into new sites and effector systems. Nat. Rev. Immunol. 14, 811–820 (2014).
Merle, N. S., Church, S. E., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system part I – molecular mechanisms of activation and regulation. Front. Immunol. 6, 262 (2015).
Smith, R. J. et al. New approaches to the treatment of dense deposit disease. J. Am. Soc. Nephrol. 18, 2447–2456 (2007).
Zipfel, P. F. & Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 9, 729–740 (2009).
Zipfel, P. F., Smith, R. J. & Skerka, C. Factor I and factor H deficiency in renal diseases: similar defects in the fluid phase have a different outcome at the surface of the glomerular basement membrane. Nephrol. Dial. Transplant. 24, 385–387 (2009).
Ricklin, D., Reis, E. S. & Lambris, J. D. Complement in disease: a defence system turning offensive. Nat. Rev. Nephrol. 12, 383–401 (2016).
Thurman, J. M., Ljubanovic, D., Edelstein, C. L., Gilkeson, G. S. & Holers, V. M. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J. Immunol. 170, 1517–1523 (2003).
Danobeitia, J. S., Djamali, A. & Fernandez, L. A. The role of complement in the pathogenesis of renal ischemia-reperfusion injury and fibrosis. Fibrogenesis Tissue Repair 7, 16 (2014).
Zou, L. et al. Complement factor B is the downstream effector of TLRs and plays an important role in a mouse model of severe sepsis. J. Immunol. 191, 5625–5635 (2013).
Miwa, T., Sato, S., Gullipalli, D., Nangaku, M. & Song, W.-C. Blocking properdin, the alternative pathway, and anaphylatoxin receptors ameliorates renal ischemia-reperfusion injury in decay-accelerating factor and CD59 double-knockout mice. J. Immunol. 190, 3552–3559 (2013).
Sethi, S. et al. Glomeruli of dense deposit disease contain components of the alternative and terminal complement pathway. Kidney Int. 75, 952–960 (2009).
Sethi, S. & Fervenza, F. C. Membranoproliferative glomerulonephritis — a new look at an old entity. New Engl. J. Med. 366, 1119–1131 (2012).
Gou, S.-J., Yuan, J., Chen, M., Yu, F. & Zhao, M.-H. Circulating complement activation in patients with anti-neutrophil cytoplasmic antibody–associated vasculitis. Kidney Int. 83, 129–137 (2013).
Vernon, K. A. & Cook, H. T. Complement in glomerular disease. Adv. Chronic Kidney Dis. 19, 84–92 (2012).
Lesher, A. M. et al. Combination of factor H mutation and properdin deficiency causes severe C3 glomerulonephritis. J. Am. Soc. Nephrol. 24, 53–65 (2013).
Zhang, Y. et al. Causes of alternative pathway dysregulation in dense deposit disease. Clin. J. Am. Soc. Nephrol. 7, 265–274 (2012).
Saraf, S. L. et al. Haemoglobinuria is associated with chronic kidney disease and its progression in patients with sickle cell anaemia. Br. J. Haematol. 164, 729–739 (2014).
Pawluczkowycz, A. W., Lindorfer, M. A., Waitumbi, J. N. & Taylor, R. P. Hematin promotes complement alternative pathway-mediated deposition of C3 activation fragments on human erythrocytes: potential implications for the pathogenesis of anemia in malaria. J. Immunol. 179, 5543–5552 (2007).
Evans, K. J., Hansen, D. S., van Rooijen, N., Buckingham, L. A. & Schofield, L. Severe malarial anemia of low parasite burden in rodent models results from accelerated clearance of uninfected erythrocytes. Blood 107, 1192–1199 (2006).
Mold, C., Tamerius, J. D. & Phillips, G. Complement activation during painful crisis in sickle cell anemia. Clin. Immunol. Immunopathol. 76, 314–320 (1995).
Chudwin, D. S., Papierniak, C., Lint, T. F. & Korenblit, A. D. Activation of the alternative complement pathway by red blood cells from patients with sickle cell disease. Clin. Immunol. Immunopathol. 71, 199–202 (1994).
Chapin, J., Terry, H. S., Kleinert, D. & Laurence, J. The role of complement activation in thrombosis and hemolytic anemias. Transfus. Apher. Sci. 54, 191–198 (2016).
Wang, R. H., Phillips, G., Medof, M. E. & Mold, C. Activation of the alternative complement pathway by exposure of phosphatidylethanolamine and phosphatidylserine on erythrocytes from sickle cell disease patients. J. Clin. Invest. 92, 1326–1335 (1993).
de Ciutiis, A., Polley, M. J., Metakis, L. J. & Peterson, C. M. Immunologic defect of the alternate pathway-of-complement activation postsplenectomy: a possible relation between splenectomy and infection. J. Natl Med. Assoc. 70, 667–670 (1978).
Ruiz-Torres, M. P. et al. Complement activation: the missing link between ADAMTS-13 deficiency and microvascular thrombosis of thrombotic microangiopathies. Thromb. Haemost. 93, 443–452 (2005).
Belcher, J. D., Marker, P. H., Weber, J. P., Hebbel, R. P. & Vercellotti, G. M. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood 96, 2451–2459 (2000).
Deuel, J. W. et al. Different target specificities of haptoglobin and hemopexin define a sequential protection system against vascular hemoglobin toxicity. Free Rad. Biol. Med. 89, 931–943 (2015).
Camus, S. M. et al. Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood 125, 3805–3814 (2015).
Roumenina, L. T., Rayes, J., Frimat, M. & Fremeaux-Bacchi, V. Endothelial cells: source, barrier, and target of defensive mediators. Immunol. Rev. 274, 307–329 (2016).
Merle, N. S., Noe, R., Halbwachs-Mecarelli, L., Fremeaux-Bacchi, V. & Roumenina, L. T. Complement system part II: role in immunity. Front. Immunol. 6, 257 (2015).
van Bijnen, S. T., Wouters, D., van Mierlo, G. J., Muus, P. & Zeerleder, S. Neutrophil activation and nucleosomes as markers of systemic inflammation in paroxysmal nocturnal hemoglobinuria: effects of eculizumab. J. Thromb. Haemost. 13, 2004–2011 (2015).
Zeerleder, S. et al. Administration of C1 inhibitor reduces neutrophil activation in patients with sepsis. Clin. Diagn. Lab. Immunol. 10, 529–535 (2003).
Martines, A. M. F. et al. Iron metabolism in the pathogenesis of iron-induced kidney injury. Nat. Rev. Nephrol. 9, 385–398 (2013).
Nath, K. A. et al. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int. 59, 106–117 (2001).
García-Camín, R. M. et al. Molecular mediators of favism-induced acute kidney injury. Clin. Nephrol. 81, 203–209 (2014).
Haase, M., Bellomo, R. & Haase-Fielitz, A. Novel biomarkers, oxidative stress, and the role of labile iron toxicity in cardiopulmonary bypass-associated acute kidney injury. J. Am. Coll. Cardiol. 55, 2024–2033 (2010).
Moreno, J. A. et al. AKI associated with macroscopic glomerular hematuria: clinical and pathophysiologic consequences. Clin. J. Am. Soc. Nephrol. 7, 175–184 (2012).
Vermeulen Windsant, I. C. et al. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front. Physiol. 5, 340 (2014).
Vermeulen Windsant, I. C. et al. Hemolysis is associated with acute kidney injury during major aortic surgery. Kidney Int. 77, 913–920 (2010).
Tracz, M. J., Alam, J. & Nath, K. A. Physiology and pathophysiology of heme: implications for kidney disease. J. Am. Soc. Nephrol. 18, 414–420 (2007).
Kanakiriya, S. K. R. et al. Heme: a novel inducer of MCP-1 through HO-dependent and HO-independent mechanisms. Am. J. Physiol. Renal Physiol. 284, F546–F554 (2003).
Nath, K. A. et al. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am. J. Pathol. 158, 893–903 (2001).
Qian, Q., Nath, K. A., Wu, Y., Daoud, T. M. & Sethi, S. Hemolysis and acute kidney failure. Am. J. Kidney Dis. 56, 780–784 (2010).
Ballarín, J. et al. Acute renal failure associated to paroxysmal nocturnal haemoglobinuria leads to intratubular haemosiderin accumulation and CD163 expression. Nephrol. Dial. Transplant. 26, 3408–3411 (2011).
Billings, F. T., Ball, S. K., Roberts, L. J. & Pretorius, M. Postoperative acute kidney injury is associated with hemoglobinemia and an enhanced oxidative stress response. Free Radic. Biol. Med. 50, 1480–1487 (2011).
Murray, R. K., Connell, G. E. & Pert, J. H. The role of haptoglobin in the clearance and distribution of extracorpuscular hemoglobin. Blood 17, 45–53 (1961).
Andersen, M. N., Mouritzen, C. V. & Gabrielli, E. R. Mechanisms of plasma hemoglobin clearance after acute hemolysis in dogs: serum haptoglobin levels and selective deposition in liver and kidney. Ann. Surg. 164, 905–912 (1966).
Eshbach, M. L., Kaur, A., Rbaibi, Y., Tejero, J. & Weisz, O. A. Hemoglobin inhibits albumin uptake by proximal tubule cells: implications for sickle cell disease. Am. J. Physiol. Cell Physiol. 312, C733–C740 (2017).
Gburek, J. et al. Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J. Am. Soc. Nephrol. 13, 423–430 (2002).
Nath, K. A. et al. Intracellular targets in heme protein-induced renal injury. Kidney Int. 53, 100–111 (1998).
Gonzalez-Michaca, L., Farrugia, G., Croatt, A. J., Alam, J. & Nath, K. A. Heme: a determinant of life and death in renal tubular epithelial cells. Am. J. Physiol. Renal Physiol. 286, F370–F377 (2004).
Shiraishi, F. et al. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am. J. Physiol. Renal Physiol. 278, F726–F736 (2000).
Homsi, E., Janino, P. & de Faria, J. B. L. Role of caspases on cell death, inflammation, and cell cycle in glycerol-induced acute renal failure. Kidney Int. 69, 1385–1392 (2006).
Singla, S. et al. Hemin causes lung microvascular endothelial barrier dysfunction by necroptotic cell death. Am. J. Respir. Cell Mol. Biol. 57, 307–314 (2017).
Sarhan, M., von Mässenhausen, A., Hugo, C., Oberbauer, R. & Linkermann, A. Immunological consequences of kidney cell death. Cell Death Dis. 9, 114 (2018).
Fervenza, F. C. et al. Induction of heme oxygenase-1 and ferritin in the kidney in warm antibody hemolytic anemia. Am. J. Kidney Dis. 52, 972–977 (2008).
Bolisetty, S. et al. Macrophage and epithelial cell H-ferritin expression regulates renal inflammation. Kidney Int. 88, 95–108 (2015).
Zarjou, A. et al. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. J. Clin. Invest. 123, 4423–4434 (2013).
Braughler, J. M., Duncan, L. A. & Chase, R. L. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J. Biol. Chem. 261, 10282–10289 (1986).
Walter, P. B. et al. Iron deficiency and iron excess damage mitochondria and mitochondrial DNA in rats. Proc. Natl Aacad. Sci. U S A 99, 2264–2269 (2002).
Baliga, R., Ueda, N. & Shah, S. V. Increase in bleomycin-detectable iron in ischaemia/reperfusion injury to rat kidneys. Biochem. J. 291, 901–905 (1993).
Haase, M., Haase-Fielitz, A. & Bellomo, R. Cardiopulmonary bypass, hemolysis, free iron, acute kidney injury and the impact of bicarbonate. Contrib. Nephrol. 165, 28–32 (2010).
Paller, M. S. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am. J. Physiol. 255, F539–F544 (1988).
Leaf, D. E. et al. Increased plasma catalytic iron in patients may mediate acute kidney injury and death following cardiac surgery. Kidney Int. 87, 1046–1054 (2015).
Leaf, D. E., Rajapurkar, M., Lele, S. S., Mukhopadhyay, B. & Waikar, S. S. Plasma catalytic iron, AKI, and death among critically ill patients. Clin. J. Am. Soc. Nephrol. 9, 1849–1856 (2014).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Linkermann, A. et al. Regulated cell death in AKI. J. Am. Soc. Nephrol. 25, 2689–2701 (2014).
Adedoyin, O. et al. Heme oxygenase-1 mitigates ferroptosis in renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 314, F702–F714 (2017).
Müller, T. et al. Necroptosis and ferroptosis are alternative cell death pathways that operate in acute kidney failure. Cell. Mol. Life Sci. 74, 3631–3645 (2017).
Deuel, J. W. et al. Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis. 7, e2064 (2016).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Kroot, J. J. C., Tjalsma, H., Fleming, R. E. & Swinkels, D. W. Hepcidin in human iron disorders: diagnostic implications. Clin. Chem. 57, 1650–1669 (2011).
Amer, J., Goldfarb, A. & Fibach, E. Flow cytometric analysis of the oxidative status of normal and thalassemic red blood cells. Cytometry Part A 60A, 73–80 (2004).
Chiou, S.-S. et al. Lipid peroxidation and antioxidative status in β-thalassemia major patients with or without hepatitis C virus infection. Clin. Chem. Lab. Med. 44, 1226–1233 (2006).
Livrea, M. A. et al. Oxidative stress and antioxidant status in beta-thalassemia major: iron overload and depletion of lipid-soluble antioxidants. Blood 88, 3608–3614 (1996).
Walter, P. B. et al. Oxidative stress and inflammation in iron-overloaded patients with β-thalassaemia or sickle cell disease. Br. J. Haematol. 135, 254–263 (2006).
Wolff, N. A. et al. Ferroportin 1 is expressed basolaterally in rat kidney proximal tubule cells and iron excess increases its membrane trafficking. J. Cell. Mol. Med. 15, 209–219 (2011).
Leonardi, P. & Ruol, A. Renal hemosiderosis in the hemolytic anemias: diagnosis by means of needle biopsy. Blood 16, 1029–1038 (1960).
Kurts, C., Panzer, U., Anders, H.-J. & Rees, A. J. The immune system and kidney disease: basic concepts and clinical implications. Nat. Rev. Immunol. 13, 738–753 (2013).
Betjes, M. G. H. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 9, 255–265 (2013).
Hato, T. & Dagher, P. C. How the innate immune system senses trouble and causes trouble. Clin. J. Am. Soc. Nephrol. 10, 1459–1469 (2015).
Kimmel, P. L. et al. Immunologic function and survival in hemodialysis patients. Kidney Int. 54, 236–244 (1998).
Pereira, B. J. et al. Plasma levels of IL-1β, TNFα and their specific inhibitors in undialyzed chronic renal failure, CAPD and hemodialysis patients. Kidney Int. 45, 890–896 (1994).
Herbelin, A., Ureña, P., Nguyen, A. T., Zingraff, J. & Descamps-Latscha, B. Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int. 39, 954–960 (1991).
Nakanishi, I. et al. Interleukin-8 in chronic renal failure and dialysis patients. Nephrol. Dial. Transplant. 9, 1435–1442 (1994).
Brockhaus, M., Bar-Khayim, Y., Gurwicz, S., Frensdorff, A. & Haran, N. Plasma tumor necrosis factor soluble receptors in chronic renal failure. Kidney Int. 42, 663–667 (1992).
Descamps-Latscha, B. et al. Balance between IL-1β, TNF-α, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J. Immunol. 154, 882–892 (1995).
van Riemsdijk-van Overbeeke, I. C. et al. TNF-α: mRNA, plasma protein levels and soluble receptors in patients on chronic hemodialysis, on CAPD and with end-stage renal failure. Clin. Nephrol. 53, 115–123 (2000).
Memoli, B. et al. Role of different dialysis membranes in the release of interleukin-6-soluble receptor in uremic patients. Kidney Int. 58, 417–424 (2000).
Bemelmans, M. H., Gouma, D. J. & Buurman, W. A. Influence of nephrectomy on tumor necrosis factor clearance in a murine model. J. Immunol. 150, 2007–2017 (1993).
Poole, S. et al. Fate of injected interleukin 1 in rats: sequestration and degradation in the kidney. Cytokine 2, 416–422 (1990).
Nath, K. A., Croatt, A. J., Haggard, J. J. & Grande, J. P. Renal response to repetitive exposure to heme proteins: chronic injury induced by an acute insult. Kidney Int. 57, 2423–2433 (2000).
Nath, K. A. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 70, 432–443 (2006).
Morimoto, K. et al. Cytoprotective role of heme oxygenase (HO)-1 in human kidney with various renal diseases. Kidney Int. 60, 1858–1866 (2001).
Shahzad, K. et al. Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int. 87, 74–84 (2015).
Martin-Rodriguez, S. et al. TLR4 and NALP3 inflammasome in the development of endothelial dysfunction in uraemia. Eur. J. Clin. Invest. 45, 160–169 (2015).
Erdei, J. et al. Induction of NLRP3 inflammasome activation by heme in human endothelial cells. Oxid. Med. Cell. Longev. 2018, 4310816 (2018).
Billings, F. T., Yu, C., Byrne, J. G., Petracek, M. R. & Pretorius, M. Heme oxygenase-1 and acute kidney injury following cardiac surgery. Cardiorenal. Med. 4, 12–21 (2014).
Merle, N. S. et al. Characterization of renal injury and inflammation in an experimental model of intravascular hemolysis. Front. Immunol. 9, 179 (2018).
Lim, Y. K. et al. Haptoglobin reduces renal oxidative DNA and tissue damage during phenylhydrazine-induced hemolysis. Kidney Int. 58, 1033–1044 (2000).
Herter, J. M., Rossaint, J., Spieker, T. & Zarbock, A. Adhesion molecules involved in neutrophil recruitment during sepsis-induced acute kidney injury. J. Innate Immun. 6, 597–606 (2014).
Singbartl, K., Green, S. A. & Ley, K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J. 14, 48–54 (2000).
Kelly, K. J., Williams, W. W., Colvin, R. B. & Bonventre, J. V. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc. Natl Acad. Sci. U S A 91, 812–816 (1994).
Takada, M., Nadeau, K. C., Shaw, G. D. & Tilney, N. L. Early cellular and molecular changes in ischemia/reperfusion injury: Inhibition by a selectin antagonist, P-selectin glycoprotein ligand-1. Transplant. Proc. 29, 1324–1325 (1997).
Nath, K. A. et al. Role of TLR4 signaling in the nephrotoxicity of heme and heme proteins. Am. J. Physiol. Renal Physiol. 314, F906–F914 (2018).
De Paepe, M. E. & Trudel, M. The transgenic SAD mouse: a model of human sickle cell glomerulopathy. Kidney Int. 46, 1337–1345 (1994).
Strauss, J., Pardo, V., Koss, M. N., Griswold, W. & McIntosh, R. M. Nephropathy associated with sickle cell anemia: an autologous immune complex nephritis. I. Studies on nature of glomerular-bound antibody and antigen identification in a patient with sickle cell disease and immune deposit glomerulonephritis. Am. J. Med. 58, 382–387 (1975).
Pardo, V., Strauss, J., Kramer, H., Ozawa, T. & McIntosh, R. M. Nephropathy associated with sickle cell anemia: an autologous immune complex nephritis. II. Clinicopathologic study of seven patients. Am. J. Med. 59, 650–659 (1975).
Roumenina, L. T. et al. A prevalent C3 mutation in aHUS patients causes a direct C3 convertase gain of function. Blood 119, 4182–4191 (2012).
Merle, N. S. et al. Intravascular hemolysis activates complement via cell-free heme and heme-loaded microvesicles. JCI Insight 3, 96910 (2018).
Thorenz, A. et al. Enhanced activation of interleukin-10, heme oxygenase-1, and AKT in C5aR2-deficient mice is associated with protection from ischemia reperfusion injury–induced inflammation and fibrosis. Kidney Int. 94, 741–755 (2018).
Gueler, F. et al. Complement 5a receptor inhibition improves renal allograft survival. J. Am. Soc. Nephrol. 19, 2302–2312 (2008).
Lerolle, N. et al. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intens. Care Med. 36, 471–478 (2010).
Solez, K., Morel-Maroger, L. & Sraer, J. D. The morphology of ‘acute tubular necrosis’ in man: analysis of 57 renal biopsies and a comparison with the glycerol model. Medicine 58, 362–376 (1979).
Ramesh, G. & Reeves, W. B. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. J. Clin. Invest. 110, 835–842 (2002).
Ramesh, G. & Reeves, W. B. TNFR2-mediated apoptosis and necrosis in cisplatin-induced acute renal failure. Am. J. Physiol. Renal Physiol. 285, F610–F618 (2003).
Faubel, S. et al. Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1β, IL-18, IL-6, and neutrophil infiltration in the kidney. J. Pharmacol. Exp. Ther. 322, 8–15 (2007).
Kelly, K. J., Meehan, S. M., Colvin, R. B., Williams, W. W. & Bonventre, J. V. Protection from toxicant-mediated renal injury in the rat with anti-CD54 antibody. Kidney Int. 56, 922–931 (1999).
Porto, B. N. et al. Heme induces neutrophil migration and reactive oxygen species generation through signaling pathways characteristic of chemotactic receptors. J. Biol. Chem. 282, 24430–24436 (2007).
Monteiro, A. P. T. et al. Leukotriene B4 mediates neutrophil migration induced by heme. J. Immunol. 186, 6562–6567 (2011).
Kelly, K. J. et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J. Clin. Invest. 97, 1056–1063 (1996).
Li, L. et al. The chemokine receptors CCR2 and CX3CR1 mediate monocyte/macrophage trafficking in kidney ischemia–reperfusion injury. Kidney Int. 74, 1526–1537 (2008).
Jang, H. R. & Rabb, H. The innate immune response in ischemic acute kidney injury. Clin. Immunol. 130, 41–50 (2009).
Li, L. & Okusa, M. D. Blocking the immune response in ischemic acute kidney injury: the role of adenosine 2A agonists. Nat. Rev. Nephrol. 2, 432–444 (2006).
Okusa, M. D. et al. A2A adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am. J. Physiol. Renal Physiol. 279, F809–F818 (2000).
Lauriat, S. & Linas, S. L. The role of neutrophils in acute renal failure. Semin. Nephrol. 18, 498–504 (1998).
Yago, T. et al. Blocking neutrophil integrin activation prevents ischemia–reperfusion injury. J. Exper. Med. 212, 1267–1281 (2015).
Melnikov, V. Y. et al. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J. Clin. Invest. 110, 1083–1091 (2002).
Thornton, M. A., Winn, R., Alpers, C. E. & Zager, R. A. An evaluation of the neutrophil as a mediator of in vivo renal ischemic-reperfusion injury. Am. J. Pathol. 135, 509–515 (1989).
Awad, A. S. et al. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 75, 689–698 (2009).
Takeda, J. et al. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell 73, 703–711 (1993).
Bessler, M. et al. Paroxysmal nocturnal haemoglobinuria (PNH) is caused by somatic mutations in the PIG-A gene. EMBO J. 13, 110–117 (1994).
Holguin, M. H., Fredrick, L. R., Bernshaw, N. J., Wilcox, L. A. & Parker, C. J. Isolation and characterization of a membrane protein from normal human erythrocytes that inhibits reactive lysis of the erythrocytes of paroxysmal nocturnal hemoglobinuria. J. Clin. Invest. 84, 7–17 (1989).
Hill, A., DeZern, A. E., Kinoshita, T. & Brodsky, R. A. Paroxysmal nocturnal haemoglobinuria. Nat. Rev. Dis. Primers 3, 17028 (2017).
Moyo, V. M., Mukhina, G. L., Garrett, E. S. & Brodsky, R. A. Natural history of paroxysmal nocturnal haemoglobinuria using modern diagnostic assays. Br. J. Haematol. 126, 133–138 (2004).
Parker, C. et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 106, 3699–3709 (2005).
Hill, A., Kelly, R. J. & Hillmen, P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood 121, 4985–4996 (2013).
Clark, D. A., Butler, S. A., Braren, V., Hartmann, R. C. & Jenkins, D. J. The kidneys in paroxysmal nocturnal hemoglobinuria. Blood 57, 83–89 (1981).
Rubin, H. Paroxysmal nocturnal hemoglobinuria with renal failure. JAMA 215, 433–436 (1971).
Roumenina, L. T. et al. Alternative complement pathway assessment in patients with atypical HUS. J. Immunol. Methods 365, 8–26 (2011).
Noris, M. & Remuzzi, G. Atypical hemolytic-uremic syndrome. N. Engl. J. Med. 361, 1676–1687 (2009).
Platt, O. S. et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N. Engl. J. Med. 330, 1639–1644 (1994).
Powars, D. R. et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann. Intern. Med. 115, 614–620 (1991).
Darbari, D. S. et al. Severe painful vaso-occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLOS ONE 8, e79923 (2013).
Nath, K. A. & Hebbel, R. P. Sickle cell disease: renal manifestations and mechanisms. Nat. Rev. Nephrol. 11, 161–171 (2015).
Guasch, A., Navarrete, J., Nass, K. & Zayas, C. F. Glomerular involvement in adults with sickle cell hemoglobinopathies: prevalence and clinical correlates of progressive renal failure. J. Am. Soc. Nephrol. 17, 2228–2235 (2006).
Day, T. G., Drasar, E. R., Fulford, T., Sharpe, C. C. & Thein, S. L. Association between hemolysis and albuminuria in adults with sickle cell anemia. Haematologica 97, 201–205 (2012).
Tejani, A. et al. Renal lesions in sickle cell nephropathy in children. Nephron 39, 352–355 (1985).
Bhathena, D. B. & Sondheimer, J. H. The glomerulopathy of homozygous sickle hemoglobin (SS) disease: morphology and pathogenesis. J. Am. Soc. Nephrol. 1, 1241–1252 (1991).
Elfenbein, I. B., Patchefsky, A., Schwartz, W. & Weinstein, A. G. Pathology of the glomerulus in sickle cell anemia with and without nephrotic syndrome. Am. J. Pathol. 77, 357–374 (1974).
Falk, R. J. et al. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N. Engl. J. Med. 326, 910–915 (1992).
Maigne, G. et al. Glomerular lesions in patients with sickle cell disease. Medicine 89, 18–27 (2010).
Saraf, S. L. et al. Progressive glomerular and tubular damage in sickle cell trait and sickle cell anemia mouse models. Transl Res. 197, 1–11 (2018).
Nath, K. A. et al. Transgenic sickle mice are markedly sensitive to renal ischemia-reperfusion injury. Am. J. Pathol. 166, 963–972 (2005).
Green, C. J. et al. The importance of iron, calcium and free radicals in reperfusion injury: an overview of studies in ischaemic rabbit kidneys. Free Radic. Res. Commun. 7, 255–264 (1989).
Kirschner, R. E. & Fantini, G. A. Role of iron and oxygen-derived free radicals in ischemia-reperfusion injury. J. Am. Coll. Surg. 179, 103–117 (1994).
Schaid, T. R. et al. Complement activation in a murine model of sickle cell disease: inhibition of vaso-occlusion by blocking C5 activation. Blood 128, 158 (2016).
Dumas, G. et al. Eculizumab salvage therapy for delayed hemolysis transfusion reaction in sickle cell disease patients. Blood 127, 1062–1064 (2016).
Schein, A., Enriquez, C., Coates, T. D. & Wood, J. C. Magnetic resonance detection of kidney iron deposition in sickle cell disease: a marker of chronic hemolysis. J. Magnet. Res. Imaging 28, 698–704 (2008).
Chonat, S. et al. Contribution of alternative complement pathway to delayed hemolytic transfusion reaction in sickle cell disease. Haematologica 103, e483–e485 (2018).
Miller, L. H., Baruch, D. I., Marsh, K. & Doumbo, O. K. The pathogenic basis of malaria. Nature 415, 673–679 (2002).
Gramaglia, I. et al. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat. Med. 12, 1417–1422 (2006).
Ferreira, A., Balla, J., Jeney, V., Balla, G. & Soares, M. P. A central role for free heme in the pathogenesis of severe malaria: the missing link? J. Mol. Med. 86, 1097–1111 (2008).
Seixas, E. et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc. Natl Acad. Sci. USA 106, 15837–15842 (2009).
Pamplona, A. et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 13, 703–710 (2007).
Plewes, K. et al. Cell-free hemoglobin mediated oxidative stress is associated with acute kidney injury and renal replacement therapy in severe falciparum malaria: an observational study. BMC Infect. Dis. 17, 313 (2017).
Elphinstone, R. E. et al. Alterations in systemic extracellular heme and hemopexin are associated with adverse clinical outcomes in ugandan children with severe malaria. J. Infect. Dis. 214, 1268–1275 (2016).
Tran, T. H. et al. Blackwater fever in southern Vietnam: a prospective descriptive study of 50 cases. Clin. Infect. Dis. 23, 1274–1281 (1996).
Sypniewska, P. et al. Clinical and laboratory predictors of death in African children with features of severe malaria: a systematic review and meta-analysis. BMC Med. 15, 147 (2017).
Ramos, S. et al. Renal control of disease tolerance to malaria. Proc. Natl Acad. Sci. U S A 116, 5681–5686 (2019).
Charache, S. et al. Hydroxyurea: effects on hemoglobin F production in patients with sickle cell anemia. Blood 79, 2555–2565 (1992).
Charache, S. et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. New Engl. J. Med. 332, 1317–1322 (1995).
Wang, W. C. et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet 377, 1663–1672 (2011).
Ware, R. E. et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet 387, 661–670 (2016).
Voskaridou, E. et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS). Blood 115, 2354–2363 (2010).
Bartolucci, P. et al. Six months of hydroxyurea reduces albuminuria in patients with sickle cell disease. J. Am. Soc. Nephrol. 27, 1847–1853 (2016).
Oksenberg, D. et al. GBT440 increases haemoglobin oxygen affinity, reduces sickling and prolongs RBC half-life in a murine model of sickle cell disease. Br. J. Haematol. 175, 141–153 (2016).
Lehrer-Graiwer, J. et al. GBT440, a potent anti-sickling hemoglobin modifier reduces hemolysis, improves anemia and nearly eliminates sickle cells in peripheral blood of patients with sickle cell disease. Blood 126, 542–542 (2015).
Howard, J. et al. A phase 1/2 ascending dose study and open-label extension study of voxelotor in patients with sickle cell disease. Blood 133, 1865–1878 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03036813 (2019).
Vichinsky, E. et al. Results from part a of the hemoglobin oxygen affinity modulation to inhibit hbs polymerization (HOPE) trial (GBT440-031), a placebo-controlled randomized study evaluating voxelotor (GBT440) in adults and adolescents with sickle cell disease. Blood 132, 505–505 (2018).
Blyden, G., Bridges, K. R. & Bronte, L. Case series of patients with severe sickle cell disease treated with voxelotor (GBT440) by compassionate access. Am. J. Hematol. https://doi.org/10.1002/ajh.25139 (2018).
Legendre, C. M. et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N. Engl. J. Med. 368, 2169–2181 (2013).
Ingoglia, G. et al. Hemopexin counteracts systolic dysfunction induced by heme-driven oxidative stress. Free Radic. Biol. Med. 108, 452–464 (2017).
Graw, J. A. et al. Haptoglobin or hemopexin therapy prevents acute adverse effects of resuscitation after prolonged storage of red cells. Circulation 134, 945–960 (2016).
Gando, S. & Tedo, I. The effects of massive transfusion and haptoglobin therapy on hemolysis in trauma patients. Surg. Today 24, 785–790 (1994).
Kanamori, Y. et al. The effects of administration of haptoglobin for hemolysis by extracorporeal circulation. Rinsho Kyobu Geka 9, 463–467 (1989).
Imaizumi, H., Tsunoda, K., Ichimiya, N., Okamoto, T. & Namiki, A. Repeated large-dose haptoglobin therapy in an extensively burned patient: case report. J. Emerg. Med. 12, 33–37 (1994).
Yoshioka, T., Sugimoto, T., Ukai, T. & Oshiro, T. Haptoglobin therapy for possible prevention of renal failure following thermal injury: a clinical study. J. Trauma 25, 281–287 (1985).
Hashimoto, K., Nomura, K., Nakano, M., Sasaki, T. & Kurosawa, H. Pharmacological intervention for renal protection during cardiopulmonary bypass. Heart Vessels 8, 203–210 (1993).
Tanaka, K. et al. Administration of haptoglobin during cardiopulmonary bypass surgery. ASAIO Trans 37, M482–M483 (1991).
Homann, B., Kult, J. & Weis, K. H. On the use of concentrated haptoglobin in the treatment of a haemolytic transfusion accident of the ABO-system (author’s transl). Anaesthesist 26, 485–488 (1977).
Horai, T., Tanaka, K. & Takeda, M. Coronary artery bypass grafting under cardiopulmonary bypass in a patient with β-thalassemia: report of a case. Surg. Today 36, 538–540 (2006).
van Swelm, R. P. L. et al. Renal handling of circulating and renal-synthesized hepcidin and its protective effects against hemoglobin–mediated kidney injury. J. Am. Soc. Nephrol. 27, 2720–2732 (2016).
Haase-Fielitz, A. et al. Urine hepcidin has additive value in ruling out cardiopulmonary bypass-associated acute kidney injury: an observational cohort study. Crit. Care 15, R186 (2011).
Ho, J. et al. Urinary hepcidin-25 and risk of acute kidney injury following cardiopulmonary bypass. Clin. J. Am. Soc. Nephrol. 6, 2340–2346 (2011).
Prowle, J. R. et al. Greater increase in urinary hepcidin predicts protection from acute kidney injury after cardiopulmonary bypass. Nephrol. Dial. Transplant 27, 595–602 (2012).
Scindia, Y. et al. Hepcidin mitigates renal ischemia-reperfusion injury by modulating systemic iron homeostasis. J. Am. Soc. Nephrol. 26, 2800–2814 (2015).
Young, G.-H. et al. Hemojuvelin modulates iron stress during acute kidney injury: improved by furin inhibitor. Antioxid. Redox Signal. 20, 1181–1194 (2014).
Hsieh, Y.-P., Huang, C.-H., Lee, C.-Y., Lin, C.-Y. & Chang, C.-C. Silencing of hepcidin enforces the apoptosis in iron-induced human cardiomyocytes. J. Occup. Med. Toxicol. 9, 11 (2014).
Farnaud, S., Patel, A. & Evans, R. W. Modelling of a metal-containing hepcidin. Biometals 19, 527–533 (2006).
Farnaud, S. et al. Identification of an iron–hepcidin complex. Biochem. J. 413, 553–557 (2008).
Gerardi, G. et al. Recombinant human hepcidin expressed in Escherichia coli isolates as an iron containing protein. Blood Cells Mol. Dis. 35, 177–181 (2005).
Roberts, L. J. Inhibition of heme protein redox cycling: reduction of ferryl heme by iron chelators and the role of a novel through-protein electron transfer pathway. Free Radic. Biol. Med. 44, 257–260 (2008).
Sheikh, N. A., Desai, T. R. & Tirgar, P. R. Investigation into iron chelating and antioxidant potential of melilotus officinalis in iron dextran induced iron overloaded Sprague–Dawley rat model. Drug Res. 66, 618–627 (2016).
Coates, T. D. Physiology and pathophysiology of iron in hemoglobin-associated diseases. Free Radic. Biol. Med. 72, 23–40 (2014).
Sánchez-González, P. D., López-Hernandez, F. J., Morales, A. I., Macías-Nuñez, J. F. & López-Novoa, J. M. Effects of deferasirox on renal function and renal epithelial cell death. Toxicol. Lett. 203, 154–161 (2011).
Groebler, L. K., Liu, J., Shanu, A., Codd, R. & Witting, P. K. Comparing the potential renal protective activity of desferrioxamine B and the novel chelator desferrioxamine B-N-(3-hydroxyadamant-1-yl)carboxamide in a cell model of myoglobinuria. Biochem. J. 435, 669–677 (2011).
Piga, A. G. et al. Deferasirox effect on renal haemodynamic parameters in patients with transfusion-dependent β thalassaemia. Br. J. Haematol. 168, 882–890 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01905774 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00560820 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01459718 (2015).
Ware, K. et al. N-acetylcysteine ameliorates acute kidney injury but not glomerular hemorrhage in an animal model of warfarin-related nephropathy. Am. J. Physiol. Renal Physiol. 304, F1421–F1427 (2013).
Zager, R. A. & Gamelin, L. M. Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am. J. Physiol. 256, F446–F455 (1989).
Zager, R. A. Studies of mechanisms and protective maneuvers in myoglobinuric acute renal injury. Lab. Invest. 60, 619–629 (1989).
Boutaud, O. & Roberts, L. J. Mechanism-based therapeutic approaches to rhabdomyolysis-induced renal failure. Free Radic. Biol. Med. 51, 1062–1067 (2011).
Huerta-Alardín, A. L., Varon, J. & Marik, P. E. Bench-to-bedside review: rhabdomyolysis – an overview for clinicians. Crit. Care 9, 158 (2004).
Groebler, L. K. et al. Cosupplementation with a synthetic, lipid-soluble polyphenol and vitamin C inhibits oxidative damage and improves vascular function yet does not inhibit acute renal injury in an animal model of rhabdomyolysis. Free Radic. Biol. Med. 52, 1918–1928 (2012).
Singh, D., Chander, V. & Chopra, K. Protective effect of naringin, a bioflavonoid on glycerol-induced acute renal failure in rat kidney. Toxicology 201, 143–151 (2004).
Rodrigo, R., Bosco, C., Herrera, P. & Rivera, G. Amelioration of myoglobinuric renal damage in rats by chronic exposure to flavonol-rich red wine. Nephrol. Dial. Transplant. 19, 2237–2244 (2004).
Chander, V., Singh, D. & Chopra, K. Reversal of experimental myoglobinuric acute renal failure in rats by quercetin, a bioflavonoid. Phramacology 73, 49–56 (2005).
Avramovic, V., Vlahovic, P., Mihailovic, D. & Stefanovic, V. Protective effect of a bioflavonoid proanthocyanidin-BP1 in glycerol-induced acute renal failure in the rat: renal stereological study. Ren. Fail. 21, 627–634 (1999).
Aydogdu, N. et al. Protective effects of L-carnitine on myoglobinuric acute renal failure in rats. Clin. Exper. Pharmacol. Physiol. 33, 119–124 (2006).
Bruno, S. et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J. Am. Soc. Nephrol. 20, 1053–1067 (2009).
Korkmaz, B., Horwitz, M. S., Jenne, D. E. & Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol. Rev. 62, 726–759 (2010).
Li, G. et al. The neutrophil elastase inhibitor, sivelestat, attenuates sepsis-related kidney injury in rats. Int. J. Mol. Med. 38, 767–775 (2016).
Huber-Lang, M. et al. Double blockade of CD14 and complement C5 abolishes the cytokine storm and improves morbidity and survival in polymicrobial sepsis in mice. J. Immunol. 192, 5324–5331 (2014).
Romay-Penabad, Z. et al. Complement C5-inhibitor rEV576 (coversin) ameliorates in-vivo effects of antiphospholipid antibodies. Lupus 23, 1324–1326 (2014).
Weston-Davies, W. H. et al. Clinical and immunological characterisation of coversin, a novel small protein inhibitor of complement C5 with potential as a therapeutic agent in PNH and other complement mediated disorders. Blood 124, 4280 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02591862?term=coversin&rank=3 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03427060 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03829449 (2019).
Kutlar, A. et al. A potent oral P-selectin blocking agent improves microcirculatory blood flow and a marker of endothelial cell injury in patients with sickle cell disease. Am. J. Hematol. 87, 536–539 (2012).
Ataga, K. I. et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N. Engl. J. Med. 376, 429–439 (2017).
Schmidt, C. Q. et al. Rational engineering of a minimized immune inhibitor with unique triple-targeting properties. J. Immunol. 190, 5712–5721 (2013).
Jäger, U. et al. Inhibition of complement C1s improves severe hemolytic anemia in cold agglutinin disease: a first-in-human trial. Blood 133, 893–901 (2019).
Graça-Souza, A. V., Arruda, M. A. B., de Freitas, M. S., Barja-Fidalgo, C. & Oliveira, P. L. Neutrophil activation by heme: implications for inflammatory processes. Blood 99, 4160–4165 (2002).
Colotta, F., Re, F., Polentarutti, N., Sozzani, S. & Mantovani, A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood 80, 2012–2020 (1992).
Lee, A., Whyte, M. K. B. & Haslett, C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukocyte Biol. 54, 283–288 (1993).
Hébert, M. J., Takano, T., Holthöfer, H. & Brady, H. R. Sequential morphologic events during apoptosis of human neutrophils. Modulation by lipoxygenase-derived eicosanoids. J. Immunol. 157, 3105–3115 (1996).
Kettritz, R. et al. Interleukin-8 delays spontaneous and tumor necrosis factor-α-mediated apoptosis of human neutrophils. Kidney Int. 53, 84–91 (1998).
Akgul, C., Moulding, D. A. & Edwards, S. W. Molecular control of neutrophil apoptosis. FEBS Lett. 487, 318–322 (2001).
Maianski, N. A. et al. Functional characterization of mitochondria in neutrophils: a role restricted to apoptosis. Cell Death Diff. 11, 143–153 (2004).
Klein, J. B. et al. Role of extracellular signal-regulated kinase and phosphatidylinositol-3 kinase in chemoattractant and LPS delay of constitutive neutrophil apoptosis. Cell. Signal. 13, 335–343 (2001).
Klein, J. B. et al. Granulocyte-macrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J. Immunol. 164, 4286–4291 (2000).
Arruda, M. A., Rossi, A. G., de Freitas, M. S., Barja-Fidalgo, C. & Graça-Souza, A. V. Heme inhibits human neutrophil apoptosis: involvement of phosphoinositide 3-kinase, MAPK, and NF-κB. J. Immunol. 173, 2023–2030 (2004).
Berends, E. T. M., Kuipers, A., Ravesloot, M. M., Urbanus, R. T. & Rooijakkers, S. H. M. Bacteria under stress by complement and coagulation. FEMS Microbiol. Rev. 38, 1146–1171 (2014).
Joiner, K. A., Brown, E. J. & Frank, M. M. Complement and bacteria: chemistry and biology in host defense. Annu. Rev. Immunol. 2, 461–491 (1984).
Sayegh, E. T., Bloch, O. & Parsa, A. T. Complement anaphylatoxins as immune regulators in cancer. Cancer Med. 3, 747–758 (2014).
Klos, A. et al. The role of the anaphylatoxins in health and disease. Mol. Immunol. 46, 2753–2766 (2009).
Ehrengruber, M. U., Geiser, T. & Deranleau, D. A. Activation of human neutrophils by C3a and C5A. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS Lett. 346, 181–184 (1994).
Elsner, J., Oppermann, M., Czech, W. & Kapp, A. C3a activates the respiratory burst in human polymorphonuclear neutrophilic leukocytes via pertussis toxin-sensitive G-proteins. Blood 83, 3324–3331 (1994).
Yuen, J. et al. NETosing neutrophils activate complement both on their own NETs and bacteria via alternative and non-alternative pathways. Front. Immunol. 7, 137 (2016).
Camous, L. et al. Complement alternative pathway acts as a positive feedback amplification of neutrophil activation. Blood 117, 1340–1349 (2011).
Martinelli, S. et al. Induction of genes mediating interferon-dependent extracellular trap formation during neutrophil differentiation. J. Biol. Chem. 279, 44123–44132 (2004).
Palmer, L. J., Damgaard, C., Holmstrup, P. & Nielsen, C. H. Influence of complement on neutrophil extracellular trap release induced by bacteria. J. Periodont. Res. 51, 70–76 (2016).
Wang, H., Wang, C., Zhao, M.-H. & Chen, M. Neutrophil extracellular traps can activate alternative complement pathways. Clin. Exp. Immunol. 181, 518–527 (2015).
Leffler, J. et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 188, 3522–3531 (2012).
Schneider, A. E., Sándor, N., Kárpáti, É. & Józsi, M. Complement factor H modulates the activation of human neutrophil granulocytes and the generation of neutrophil extracellular traps. Mol. Immunol. 72, 37–48 (2016).
O’Flynn, J., Dixon, K. O., Faber Krol, M. C., Daha, M. R. & van Kooten, C. Myeloperoxidase directs properdin-mediated complement activation. J. Innate Immun. 6, 417–425 (2014).
Dhalla, N. S., Elmoselhi, A. B., Hata, T. & Makino, N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc. Res. 47, 446–456 (2000).
Sponsel, H. T. et al. Effect of iron on renal tubular epithelial cells. Kidney Int. 50, 436–444 (1996).
Zager, R. A., Johnson, A. C. M. & Becker, K. Renal cortical hemopexin accumulation in response to acute kidney injury. Am. J. Physiol. Renal Physiol. 303, F1460–F1472 (2012).
Sears, D. A., Anderson, P. R., Foy, A. L., Williams, H. L. & Crosby, W. H. Urinary iron excretion and renal metabolism of hemoglobin in hemolytic diseases. Blood 28, 708–725 (1966).
Zager, R. A., Foerder, C. & Bredl, C. The influence of mannitol on myoglobinuric acute renal failure: functional, biochemical, and morphological assessments. J. Am. Soc. Nephrol. 2, 848–855 (1991).
Veuthey, T., D’Anna, M. C. & Roque, M. E. Role of the kidney in iron homeostasis: renal expression of prohepcidin, ferroportin, and DMT1 in anemic mice. Am. J. Physiol. Renal Physiol. 295, F1213–F1221 (2008).
Zager, R. A., Vijayan, A. & Johnson, A. C. Proximal tubule haptoglobin gene activation is an integral component of the acute kidney injury “stress response”. Am. J. Physiol. Renal Physiol. 303, F139–F148 (2012).
Hsiao, P.-J., Wang, S.-C., Wen, M.-C., Diang, L.-K. & Lin, S.-H. Fanconi syndrome and CKD in a patient with paroxysmal nocturnal hemoglobinuria and hemosiderosis. Am. J. Kidney Dis. 55, e1–e5 (2010).
Shah, S. V., Baliga, R., Rajapurkar, M. & Fonseca, V. A. Oxidants in chronic kidney disease. J. Am. Soc. Nephrol. 18, 16–28 (2007).
Author information
Authors and Affiliations
Contributions
K.V.A. researched data for and wrote the article. All authors discussed the article’s content and reviewed/edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Nephrology thanks G. Vercellotti and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Glossary
- Sickle cell disease
-
(SCD). A β-globin disorder driven by haemoglobin S polymerization and characterized by recurrent vaso-occlusive episodes, the propensity of red blood cells to sickle and neutrophil recruitment. SCD alters renal function and causes various renal manifestations (sickle cell nephropathy).
- Thalassaemias
-
Common inherited monogenic haemoglobin disorders, characterized by an imbalance in the ratio of α-globin to β-globin chains, chronic haemolytic anaemia, ineffective erythropoiesis, compensatory haematopoietic expansion, a state of hypercoagulability and iron overload.
- Spherocytosis
-
Hereditary spherocytosis is a type of congenital haemolytic anaemia that results from genetic mutations in red blood cell membrane or cytoskeletal proteins involved in morphological stability and affects the ability of red blood cells to maintain their normal biconcave shape. The clinical manifestations vary based on the severity of disease and the type of mutation.
- Paroxysmal nocturnal haemoglobinuria
-
(PNH). A clonal haematopoietic disease caused by expansion of a stem cell that harbours a somatic mutation in PIGA. Red blood cells that harbour the mutation are deficient in the complement regulator proteins CD55 and CD59, increasing their susceptibility to intravascular haemolysis due to a failure to regulate the alternative pathway of complement.
- Autoimmune haemolytic anaemia
-
(AIHA). Caused by autoantibodies against red blood cells that can fix complement on the red blood cell surface and trigger haemolysis at excessive or uncompensated rates.
- Thrombotic microangiopathies
-
(TMA). A diverse group of syndromes that can be hereditary or acquired, characterized by vascular damage and capillary thrombosis with typical abnormalities in the endothelium and vessel wall. Clinical features include microangiopathic haemolytic anaemia and a procoagulant state, with or without damage to the kidneys and other organs.
- Haemolytic transfusion reaction
-
A clinical situation that involves transfusion-related ABO incompatibility with brisk complement-mediated lysis of red blood cells.
- Chemically induced anaemia
-
A form of extracorpuscular haemolytic disorder caused by exposure to chemical substances. Haemolytic anaemia induced by chemical agents might be particularly detrimental for people with hereditable haemolytic disorders (e.g. G6PD deficiency) and for people with pre-existing anaemia.
- Haemoglobin H disease
-
The most severe non-fatal form of α-thalassaemia, which occurs when only one normal α-globin gene has been inherited (compound heterozygosity). An insufficiency of α-globin chains results in inadequate formation of HbA (α2β2), resulting in an excess of β-globin chains, which become unstable and precipitate as HbH (β4), causing haemolysis.
- Schizont
-
A stage of the Plasmodium spp. life cycle that occurs following infection of red blood cells with Plasmodium merozoites. The schizont contains 16–32 merozoites and occurs within 40–48 hours of infection. Red blood cell egress releases merozoites into the bloodstream, where the cycle recommences.
- Haemoproteins
-
Proteins that contain a haem prosthetic group and are responsible for containing more than 80% of bioavailable iron in mammals. Based on the capacity of haem-iron to exchange electrons, haemoproteins play an essential role in a variety of vital cellular functions: the major haemoprotein pool in mammals is formed by haemoglobin in red blood cells and myoglobin in muscle cells. Another important compartment involves ubiquitously expressed cytochromes.
- Fenton reaction
-
A reaction that involves the transition of haem-iron from a ferrous to ferric state, generating Hb-Fe3+ (methaemoglobin) and highly reactive hydroxyl radicals. The participation of iron in the production of free radicals via this reaction is potentially cytotoxic.
- Favism
-
By far the most identifiable cause of acute haemolytic anaemia caused by glucose-6-phosphate dehydrogenase (G6PD) deficiency. Haemolysis of G6PD-deficient red blood cells (RBCs) arises because nicotinamide adenine dinucleotide phosphate (NADPH) generation is insufficient to provide antioxidant defence. Following exposure to highly reactive redox compounds released on ingestion of fava beans, G6PD-deficient RBCs cannot withstand the oxidative attack by fava bean glucosides, causing RBC destruction.
- Haemin
-
Haemin can be generated by haem oxidation, and consists of a protoporphyrin IX ring surrounding a single coordinated ferric iron (Fe3+) moiety instead of Fe2+.
- Ferritin
-
Multimeric complexes (~450 kDa) made of ferritin H (heavy) and ferritin L (light) chains. These heteropolymeric nanocage-like structures have ferroxidase activity that catalyses the conversion of Fe2+ into ferric Fe3+, allowing for intracellular storage and neutralization of inert ferric iron.
- Ferroportin 1
-
A 62 kDa transmembrane iron transporter that exports ferrous iron from cells when it accumulates above a threshold level. It is regulated by the iron regulatory hormone hepcidin, which binds ferroportin and induces its internalization and degradation.
- Haemosiderosis
-
Also known as iron storage disease, characterized by accelerated haemosiderin deposition at high cellular iron storage levels, causing an irreversible iron overload.
- Infiltrating neutrophils
-
In the circulation, neutrophils patrol tissues and drive aggressive responses after exposure to danger signals (sterile or triggered by microorganisms). This is accomplished by swift transmigration into the damaged tissue, where infiltrating neutrophils release reactive chemicals and proteases.
- Marginating neutrophils
-
Although intravascular, this pool of neutrophils remains reversibly but intimately associated with the endothelium, resisting the shearing forces of flowing blood.
- Clonal haematopoiesis
-
Uncontrolled expansion and clonal selection of one or more haematopoietic stem cells results in clonal evolution, which can compete with and — as described for PNH —overcome normal haematopoiesis. Selection for specific clones is most likely related to certain somatic mutations at the stem cell level or to changes in the haematopoietic niche.
- Haemosiderin
-
The non-ferritin constituent of iron stores, which accumulates as insoluble, aggregated deposits as the amount of cellular iron increases. It has no physiological role in iron metabolism but can sequester cellular iron waste.
- Acute chest syndrome
-
A form of acute lung injury and the second most common cause of hospital admission in patients with sickle cell disease, in whom it is associated with high mortality. It is caused by a combination of infection, fat embolism and vaso-occlusion of the pulmonary vasculature with subsequent development of a new alveolar pulmonary infiltrate.
Rights and permissions
About this article
Cite this article
Van Avondt, K., Nur, E. & Zeerleder, S. Mechanisms of haemolysis-induced kidney injury. Nat Rev Nephrol 15, 671–692 (2019). https://doi.org/10.1038/s41581-019-0181-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0181-0
This article is cited by
-
FGF21 Inhibits Hypoxia/Reoxygenation-induced Renal Tubular Epithelial Cell Injury by Regulating the PPARγ/NF-κB Signaling Pathway
Cell Biochemistry and Biophysics (2024)
-
Iron as an emerging therapeutic target in critically ill patients
Critical Care (2023)
-
Acute kidney injury in hospitalized children with sickle cell anemia
BMC Nephrology (2022)
-
Blackwater fever and acute kidney injury in children hospitalized with an acute febrile illness: pathophysiology and prognostic significance
BMC Medicine (2022)
-
Modeling oxidative injury response in human kidney organoids
Stem Cell Research & Therapy (2022)