Airlines are among the companies keen on converting biomass to liquid fuel.Credit: Arseniy Shemyakin Photo/Shutterstock
Necessity is the mother of invention, and a century ago, nations needed petroleum. They could run ships on coal, but burning solid lumps of fuel was impractical for cars and tanks, and unsuited to aircraft. Unlike other countries, Germany had no access to crude oil, so two chemists there — Franz Fischer and Hans Tropsch — invented a way to make synthetic petroleum from coal in 1925.
Their Fischer–Tropsch (FT) process could now help countries and companies that want to phase out fossil fuels: if coal can be turned into liquid fuels, then, theoretically, greener alternatives such as biomass could be as well. But so far, efforts to do this have been inefficient, and certainly not cheap enough to compete with oil.
Read the paper: Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology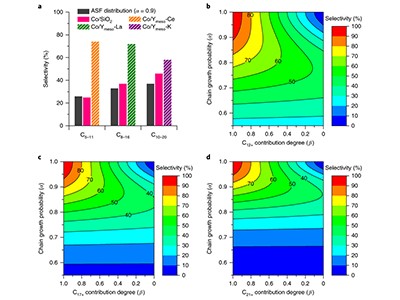
A study in Nature Catalysis this week points to a possible way forward. Chemists in Japan and China have boosted the FT process, and improved on how it can be steered to produce different liquid fuels (J. Li et al. Nature Catal. http://doi.org/ctxv; 2018).
Although the FT process is good at converting gases — used directly, or produced from solids such as coal or even ground-up peanut shells — it’s rather unfussy about what it churns out. Mostly, that’s a blend of synthetic-petroleum products, from light gases such as methane through to heavy waxes (think Vaseline). The most useful stuff, such as petrol, diesel and aviation fuel (kerosene), falls somewhere in the middle, and must be separated and purified. That typically makes large-scale FT synthesis of those fuels a two-step process, which increases costs, complexity and pollution.
As a consequence, it’s usually used commercially to make synthetic liquid fuels only where the feedstock is unusually cheap (China operates some facilities that process coal), or where there is no alternative (the South African company Sasol developed an FT process to liquefy coal when access to foreign oil was denied by sanctions in the apartheid era).
The latest study shows that this conversion can be made more selective. With small tweaks to the composition of the catalyst used — a well-known porous material, called a zeolite, mixed with cobalt nanoparticles — the team steered the chemical reaction to produce significant quantities of the desired liquid fuel. For example, the chemists could tune it to make 74% pure petrol (gasoline) or 72% pure jet fuel. Conventionally, it was difficult to produce anything more than 50% using FT synthesis, in a process usually based on iron or cobalt catalysts supported on silica or aluminium oxide. This is one of a string of recent results to show that barrier can be overcome.
There remains some way to go. Zeolite-based catalysts are notorious for their fast deactivation, and the paper reports the synthesis of the fuels in a reactor the size of a thimble, using just a single gram of catalyst. To make it economical, the process would need to be run stably for much longer and scaled up to much larger reactors using at least 100 tonnes of catalyst. Enthusiasm for synthetic fuels ebbs and flows with the market: they were popular a decade ago when oil prices were at record levels, but not so much now. There is no guarantee that the market demand for these fuels will drive the necessary investment.
Noritatsu Tsubaki, a chemist at the University of Toyama in Japan who led the project, says a major advantage of the process is that it could be used to make ‘one-step’ direct synthesis of kerosene and petrol from FT reactions for the first time — with yields high enough to avoid needing the separation step. Several airlines are already looking into FT chemistry as a source of fuel, and Tsubaki says his team plans to contact airlines and aircraft manufacturers with the findings. The necessity is clearly there, and now, so is a possible invention.

 Read the paper: Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology
Read the paper: Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology
 Cobalt gets in shape
Cobalt gets in shape
 Cobalt carbide nanoprisms for direct production of lower olefins from syngas
Cobalt carbide nanoprisms for direct production of lower olefins from syngas