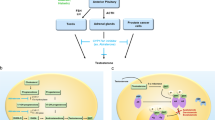
Abstract
Radiotherapy in combination with androgen deprivation therapy (ADT) is a standard treatment option for men with localized and locally advanced prostate cancer. However, emerging clinical evidence suggests that radiotherapy can be incorporated into multimodality therapy regimens beyond ADT, in combinations that include chemotherapy, radiosensitizing agents, immunotherapy and surgery for the treatment of men with localized and locally advanced prostate cancer, and those with oligometastatic disease, in whom the low metastatic burden in particular might be treatable with these combinations. This multimodal approach is increasingly recognized as offering considerable clinical benefit, such as increased antitumour effects and improved survival. Thus, radiotherapy is becoming a key component of multimodal therapy for many stages of prostate cancer, particularly oligometastatic disease.
Key points
-
Radiotherapy combined with androgen deprivation therapy (ADT) is a common treatment option for men with prostate cancer.
-
In many patients, the use of radiotherapy is hindered by tumour resistance and a reduction in quality of life caused by toxic effects to normal tissue, which limits the radiation dose that can be delivered safely.
-
Tumour control could be improved by using radiotherapy in combination with other treatments beyond ADT, including chemotherapy, radiosensitizing agents, immunotherapy and surgery.
-
This multimodality approach could be beneficial in men with many stages of prostate cancer, including oligometastatic disease.
-
A survival benefit from using a multimodality therapy approach seems to be achievable in the context of the low metastatic burden of oligometastatic prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ferlay, J. et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 103, 356–387 (2018).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 69, 7–34 (2019).
Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 10, 63–89 (2019).
Rodrigues, G. et al. Pre-treatment risk stratification of prostate cancer patients: a critical review. Can. Urol. Assoc. J. 6, 121–127 (2012).
D’Amico, A. V. et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280, 969–974 (1998).
Bastian, P. J. et al. High-risk prostate cancer: from definition to contemporary management. Eur. Urol. 61, 1096–1106 (2012).
Heidenreich, A. et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur. Urol. 65, 124–137 (2014).
Mottet, N. et al. EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol. 71, 618–629 (2017).
Heidenreich, A. et al. EAU guidelines on prostate cancer. part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol. 65, 467–479 (2014).
Sanda, M. G. et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. part I: risk stratification, shared decision making, and care options. J. Urol. 199, 683–690 (2018).
Sanda, M. G. et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. part II: recommended approaches and details of specific care options. J. Urol. 199, 990–997 (2018).
Chang, A. J., Autio, K. A., Roach, M. & Scher, H. I. High-risk prostate cancer: classification and therapy. Nat. Rev. Clin. Oncol. 11, 308–323 (2014).
Cooper, B. T. & Sanfilippo, N. J. Concurrent chemoradiation for high-risk prostate cancer. World J. Clin. Oncol. 6, 35–42 (2015).
Bolla, M. et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N. Engl. J. Med. 337, 295–300 (1997).
Jones, C. U. et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N. Engl. J. Med. 365, 107–118 (2011).
Horwitz, E. M. et al. Ten-year follow-up of Radiation Therapy Oncology Group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J. Clin. Oncol. 26, 2497–2504 (2008).
Parker, C. C. et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet 392, 2353–2366 (2018).
Palma, D. A. et al. The oligometastatic state–separating truth from wishful thinking. Nat. Rev. Clin. Oncol. 11, 549–557 (2014).
Tosoian, J. J. et al. Oligometastatic prostate cancer: definitions, clinical outcomes and treatment considerations. Nat. Rev. Urol. 14, 15–25 (2017).
Ost, P. et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J. Clin. Oncol. 36, 446–453 (2018).
Palma, D. A. et al. Stereotactic ablative radiation therapy for the comprehensive treatment of oligometastatic tumors (SABR-COMET): results of a randomized trial. Int. J. Radiat. Oncol. 102, S3–S4 (2018).
Lussier, Y. A. et al. MicroRNA expression characterizes oligometastasis(es). PLoS ONE 6, e28650 (2011).
Wuttig, D. et al. Gene signatures of pulmonary metastases of renal cell carcinoma reflect the disease-free interval and the number of metastases per patient. Int. J. Cancer 125, 474–482 (2009).
Hong, M. K. H. et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nat. Commun. 6, 6605 (2015).
Sathianathen, N. J. et al. The phytological future of prostate cancer staging: PSMA-PET and the dandelion theory. Future Oncol. 13, 1801–1807 (2017).
Perera, M. et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur. Urol. 70, 926–937 (2016).
Li, R. et al. The use of PET/CT in prostate cancer. Prostate Cancer Prostatic Dis. 21, 4–21 (2018).
Stevens, D. J. & Sooriakumaran, P. Oligometastatic prostate cancer. Curr. Treat. Options Oncol. 17, 62 (2016).
Khoo, V. Is there another bite of the cherry? The case for radical local therapy for oligometastatic disease in prostate cancer. Eur. Urol. 69, 13–14 (2016).
Sooriakumaran, P. Testing radical prostatectomy in men with prostate cancer and oligometastases to the bone: a randomized controlled feasibility trial. BJU Int. 120, E8–E20 (2017).
Rusthoven, C. G. et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J. Clin. Oncol. 34, 2835–2842 (2016).
Lardas, M. et al. Quality of life outcomes after primary treatment for clinically localised prostate cancer: a systematic review. Eur. Urol. 72, 869–885 (2017).
Gaither, T. W. et al. The natural history of erectile dysfunction after prostatic radiotherapy: a systematic review and meta-analysis. J. Sex. Med. 14, 1071–1078 (2017).
Matta, R. et al. Pelvic complications after prostate cancer radiation therapy and their management: an international collaborative narrative review. Eur. Urol. 75, 464–476 (2019).
Matzinger, O. et al. Acute toxicity of curative radiotherapy for intermediate- and high-risk localised prostate cancer in the EORTC trial 22991. Eur. J. Cancer 45, 2825–2834 (2009).
Steel, G. G., McMillan, T. J. & Peacock, J. H. The 5Rs of radiobiology. Int. J. Radiat. Biol. 56, 1045–1048 (1989).
Bernier, J., Hall, E. J. & Giaccia, A. Radiation oncology: a century of achievements. Nat. Rev. Cancer 4, 737–747 (2004).
Lomax, M. E., Folkes, L. K. & O’Neill, P. Biological consequences of radiation-induced DNA damage: relevance to radiotherapy. Clin. Oncol. 25, 578–585 (2013).
Durante, M. & Loeffler, J. S. Charged particles in radiation oncology. Nat. Rev. Clin. Oncol. 7, 37–43 (2010).
Loeffler, J. S. & Durante, M. Charged particle therapy-optimization, challenges and future directions. Nat. Rev. Clin. Oncol. 10, 411–424 (2013).
Eriksson, D. & Stigbrand, T. Radiation-induced cell death mechanisms. Tumour Biol. 31, 363–372 (2010).
Vakifahmetoglu, H., Olsson, M. & Zhivotovsky, B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 15, 1153–1162 (2008).
Baskar, R., Lee, K. A., Yeo, R. & Yeoh, K.-W. Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci. 9, 193–199 (2012).
Golden, E. B., Pellicciotta, I., Demaria, S., Barcellos-Hoff, M. H. & Formenti, S. C. The convergence of radiation and immunogenic cell death signaling pathways. Front. Oncol. 2, 88 (2012).
Galluzzi, L., Buqué, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Golden, E. B. & Apetoh, L. Radiotherapy and immunogenic cell death. Semin. Radiat. Oncol. 25, 11–17 (2015).
Brown, J. M., Carlson, D. J. & Brenner, D. J. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int. J. Radiat. Oncol. Biol. Phys. 88, 254–262 (2014).
Jeong, H., Bok, S., Hong, B.-J., Choi, H.-S. & Ahn, G.-O. Radiation-induced immune responses: mechanisms and therapeutic perspectives. Blood Res. 51, 157–163 (2016).
Janiak, M. K., Wincenciak, M., Cheda, A., Nowosielska, E. M. & Calabrese, E. J. Cancer immunotherapy: how low-level ionizing radiation can play a key role. Cancer Immunol. Immunother. 66, 819–832 (2017).
Barker, H. E., Paget, J. T. E., Khan, A. A. & Harrington, K. J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer. 15, 409–425 (2015).
Zaorsky, N. G. et al. The evolution of brachytherapy for prostate cancer. Nat. Rev. Urol. 14, 415–439 (2017).
Connell, P. P. & Hellman, S. Advances in radiotherapy and implications for the next century: a historical perspective. Cancer Res. 69, 383–392 (2009).
Otto, K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med. Phys. 35, 310–317 (2008).
Palma, D. et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 72, 996–1001 (2008).
Ren, W. et al. Dosimetric comparison of intensity-modulated radiotherapy and volumetric-modulated arc radiotherapy in patients with prostate cancer: a meta-analysis. J. Appl. Clin. Med. Phys. 17, 254–262 (2016).
Ballhausen, H., Li, M., Ganswindt, U. & Belka, C. Shorter treatment times reduce the impact of intra-fractional motion: a real-time 4DUS study comparing VMAT vs. step-and-shoot IMRT for prostate cancer. Strahlenther. Onkol. 194, 664–674 (2018).
Michalski, J. M. et al. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int. J. Radiat. Oncol. 87, 932–938 (2013).
Cahlon, O., Hunt, M. & Zelefsky, M. J. Intensity-modulated radiation therapy: supportive data for prostate cancer. Semin. Radiat. Oncol. 18, 48–57 (2008).
Rubio, C., Morera, R., Hernando, O., Leroy, T. & Lartigau, S. E. Extracranial stereotactic body radiotherapy. Review of main SBRT features and indications in primary tumors. Rep. Pract. Oncol. Radiother. 18, 387–396 (2013).
Mahase, S. S. et al. Trends in the use of stereotactic body radiotherapy for treatment of prostate cancer in the United States. JAMA Netw. Open 3, e1920471 (2020).
Zelefsky, M. J. et al. Five-year outcomes of a phase 1 dose-escalation study using stereotactic body radiosurgery for patients with low-risk and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 104, 42–49 (2019).
Koskela, K. et al. Hypofractionated stereotactic body radiotherapy for localized prostate cancer – first Nordic clinical experience. Acta Oncol. 56, 978–983 (2017).
Quon, H. C. et al. Dose-escalated stereotactic body radiation therapy for prostate cancer: quality-of-life comparison of two prospective trials. Front. Oncol. 6, 185 (2016).
Kotecha, R. et al. Dose-escalated stereotactic body radiation therapy for patients with intermediate- and high-risk prostate cancer: initial dosimetry analysis and patient outcomes. Int. J. Radiat. Oncol. Biol. Phys. 95, 960–964 (2016).
Hickey, B. E., James, M. L., Daly, T., Soh, F. Y. & Jeffery, M. Hypofractionation for clinically localized prostate cancer. Cochrane Database Syst. Rev. 9, CD011462 (2019).
Kothari, G. et al. Stereotactic body radiotherapy for primary prostate cancer. Technol. Cancer Res. Treat. 17, 1533033818789633 (2018).
King, C. R. et al. Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother. Oncol. 109, 217–221 (2013).
King, C. R. et al. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: results from a multi-institutional consortium of prospective trials. Int. J. Radiat. Oncol. Biol. Phys. 87, 939–945 (2013).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01584258 (2015).
Widmark, A. et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 394, 385–395 (2019).
Viani, G. A., Stefano, E. J. & Afonso, S. L. Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int. J. Radiat. Oncol. Biol. Phys. 74, 1405–1418 (2009).
Lips, I. M. et al. Single blind randomized phase III trial to investigate the benefit of a focal lesion ablative microboost in prostate cancer (FLAME-trial): study protocol for a randomized controlled trial. Trials 12, 255 (2011).
Lim, C. et al. Magnetic resonance for radiotherapy management and treatment planning in prostatic carcinoma. Br. J. Radiol. 88, 20150507 (2015).
BioMed Central. ISRCTN registry http://www.isrctn.com/ISRCTN80146950 (2019).
Dess, R. T. et al. The current state of randomized clinical trial evidence for prostate brachytherapy. Urol. Oncol. 39, 299–610 (2019).
Vanneste, B. G. L., Van Limbergen, E. J., van Lin, E. N., van Roermund, J. G. H. & Lambin, P. Prostate cancer radiation therapy: what do clinicians have to know? Biomed. Res. Int. 2016, 6829875 (2016).
Morris, W. J. et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT Trial): an analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 98, 275–285 (2017).
Hoskin, P. J. et al. Dosimetric predictors of biochemical control of prostate cancer in patients randomised to external beam radiotherapy with a boost of high dose rate brachytherapy. Radiother. Oncol. 110, 110–113 (2014).
Hoskin, P. J. et al. Randomised trial of external beam radiotherapy alone or combined with high-dose-rate brachytherapy boost for localised prostate cancer. Radiother. Oncol. 103, 217–222 (2012).
Wisenbaugh, E. S. et al. Proton beam therapy for localized prostate cancer 101: basics, controversies, and facts. Rev. Urol. 16, 67–75 (2014).
Royce T. J. & Efstathiou J. A. Proton therapy for prostate cancer: a review of the rationale, evidence, and current state. Urol. Oncol. 37, 628–636 (2019).
The Lancet Oncology. Proton therapy for prostate cancer: time for evidence. Lancet Oncol. 15, 775 (2014).
Zhang, X. et al. Effect of anatomic motion on proton therapy dose distributions in prostate cancer treatment. Int. J. Radiat. Oncol. Biol. Phys. 67, 620–629 (2007).
Trofimov, A. et al. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int. J. Radiat. Oncol. Biol. Phys. 69, 444–453 (2007).
Sheets, N. C. et al. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307, 1611–1620 (2012).
Fang, P. et al. A case-matched study of toxicity outcomes after proton therapy and intensity-modulated radiation therapy for prostate cancer. Cancer 121, 1118–1127 (2015).
US National Library of Medicine. ClinicalTrials.gov https://www.clinicaltrials.gov/ct2/show/NCT01617161 (2019).
Franke, A. J. et al. Total neoadjuvant therapy: a shifting paradigm in locally advanced rectal cancer management. Clin. Colorectal Cancer. 17, 1–12 (2018).
van der Wilk, B. J. et al. The optimal neoadjuvant treatment of locally advanced esophageal cancer. J. Thorac. Dis. 11 (Suppl. 5), S621–S631 (2019).
Akre, O. et al. Mortality among men with locally advanced prostate cancer managed with noncurative intent: a nationwide study in PCBaSe Sweden. Eur. Urol. 60, 554–563 (2011).
Spahn, M. et al. Long-term outcome of patients with high-risk prostate cancer following radical prostatectomy and stage-dependent adjuvant androgen deprivation. Urol. Int. 84, 164–173 (2010).
Bach, C. et al. The status of surgery in the management of high-risk prostate cancer. Nat. Rev. Urol. 11, 342–351 (2014).
Johnston, T. J. et al. Mortality among men with advanced prostate cancer excluded from the ProtecT trial. Eur. Urol. 71, 381–388 (2017).
Cookson, M. S. et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 177, 540–545 (2007).
Cornford, P. et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 71, 630–642 (2017).
National Institute for Health and Care Excellence. Prostate cancer: diagnosis and management. https://www.nice.org.uk/guidance/ng131 (2019).
Bolla, M. et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 380, 2018–2027 (2012).
Thompson, I. M. et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J. Urol. 181, 956–962 (2009).
Wiegel, T. et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J. Clin. Oncol. 27, 2924–2930 (2009).
Daly, T., Hickey, B. E., Lehman, M., Francis, D. P. & See, A. M. Adjuvant radiotherapy following radical prostatectomy for prostate cancer. Cochrane Database Syst. Rev. 7, CD007234 (2011).
Parker, C. et al. Radiotherapy and androgen deprivation in combination after local surgery (RADICALS): a new Medical Research Council/National Cancer Institute of Canada phase III trial of adjuvant treatment after radical prostatectomy. BJU Int. 99, 1376–1379 (2007).
Pearse, M. et al. A phase III trial to investigate the timing of radiotherapy for prostate cancer with high-risk features: background and rationale of the Radiotherapy – Adjuvant Versus Early Salvage (RAVES) trial. BJU Int. 113, 7–12 (2014).
Parker, C. et al. Timing of radiotherapy (RT) after radical prostatectomy (RP): first results from the RADICALS RT randomised controlled trial (RCT) [NCT00541047] [abstract LBA49_PR]. Ann. Oncol. 30 (Suppl. 5), v883–v884 (2019).
Vale, C. L. et al. LBA48_PRAdjuvant or salvage radiotherapy for the treatment of localised prostate cancer? A prospectively planned aggregate data meta-analysis [abstract LBA48_PR]. Ann Oncol. 30 (Suppl. 5), v883 (2019).
Pollack, A. et al. Short term androgen deprivation therapy without or with pelvic lymph node treatment added to prostate bed only salvage radiotherapy: the NRG oncology/RTOG 0534 SPPORT trial [abstract LBA5]. Int. J. Radiat. Oncol. 102, 1605 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00667069 (2018).
Brawer, M. K. Androgen deprivation therapy: a cornerstone in the treatment of advanced prostate cancer. Rev. Urol. 6, S3–S9 (2004).
Schröder, F., Crawford, E. D., Axcrona, K., Payne, H. & Keane, T. E. Androgen deprivation therapy: past, present and future. BJU Int. 109, 1–12 (2012).
Payne, H. & Mason, M. Androgen deprivation therapy as adjuvant/neoadjuvant to radiotherapy for high-risk localised and locally advanced prostate cancer: recent developments. Br. J. Cancer 105, 1628–1634 (2011).
Leal, F., de Figueiredo, M. A. N. & Sasse, A. D. Optimal duration of androgen deprivation therapy following radiation therapy in intermediate- or high-risk nonmetastatic prostate cancer: a systematic review and meta-analysis. Int. Braz. J. Urol. 41, 425–434 (2015).
Pisansky, T. M. et al. Duration of androgen suppression before radiotherapy for localized prostate cancer: Radiation Therapy Oncology Group randomized clinical trial 9910. J. Clin. Oncol. 33, 332–339 (2015).
Nabid, A. et al. Duration of androgen deprivation therapy in high-risk prostate cancer: a randomized phase III trial. Eur. Urol. 74, 432–441 (2018).
Bolla, M. et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 360, 103–106 (2002).
Bolla, M. et al. Duration of androgen suppression in the treatment of prostate cancer. N. Engl. J. Med. 360, 2516–2527 (2009).
D’Amico, A. V. et al. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA 292, 821–827 (2004).
Bolla, M. et al. Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: results of EORTC trial 22991. J. Clin. Oncol. 34, 1748–1756 (2016).
Bolla, M. et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 11, 1066–1073 (2010).
Isbarn, H. et al. Androgen deprivation therapy for the treatment of prostate cancer: consider both benefits and risks. Eur. Urol. 55, 62–75 (2009).
Kucway, R. et al. Prostate volume reduction with androgen deprivation therapy before interstitial brachytherapy. J. Urol. 167, 2443–2447 (2002).
Milecki, P., Martenka, P., Antczak, A. & Kwias, Z. Radiotherapy combined with hormonal therapy in prostate cancer: the state of the art. Cancer Manag. Res. 2, 243–253 (2010).
Dal Pra, A., Locke, J. A., Borst, G., Supiot, S. & Bristow, R. G. Mechanistic insights into molecular targeting and combined modality therapy for aggressive, localized prostate cancer. Front. Oncol. 6, 24 (2016).
Polkinghorn, W. R. et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 3, 1245–1253 (2013).
Goodwin, J. F. et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 3, 1254–1271 (2013).
Bartek, J., Mistrik, M. & Bartkova, J. Androgen receptor signaling fuels DNA repair and radioresistance in prostate cancer. Cancer Discov. 3, 1222–1224 (2013).
Spratt, D. E. et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res. 75, 4688–4696 (2015).
Milosevic, M. et al. Androgen withdrawal in patients reduces prostate cancer hypoxia: implications for disease progression and radiation response. Cancer Res. 67, 6022–6025 (2007).
Schmidt-Hansen, M., Hoskin, P., Kirkbride, P., Hasler, E. & Bromham, N. Hormone and radiotherapy versus hormone or radiotherapy alone for non-metastatic prostate cancer: a systematic review with meta-analyses. Clin. Oncol. 26, e21–e46 (2014).
Sandler, H. M. et al. A phase III protocol of androgen suppression (AS) and 3DCRT/IMRT versus AS and 3DCRT/IMRT followed by chemotherapy (CT) with docetaxel and prednisone for localized, high-risk prostate cancer (RTOG 0521) [abstract]. J. Clin. Oncol. 33 (Suppl. 18), LBA5002 (2015).
James, N. D. et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387, 1163–1177 (2016).
Kyriakopoulos, C. E. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J. Clin. Oncol. 36, 1080–1087 (2018).
Damodaran, S., Kyriakopoulos, C. E. & Jarrard, D. F. Newly diagnosed metastatic prostate cancer: has the paradigm changed? Urol. Clin. North. Am. 44, 611–621 (2017).
Seiwert, T. Y., Salama, J. K. & Vokes, E. E. The concurrent chemoradiation paradigm–general principles. Nat. Clin. Pract. Oncol. 4, 86–100 (2015).
Moding, E. J., Kastan, M. B. & Kirsch, D. G. Strategies for optimizing the response of cancer and normal tissues to radiation. Nat. Rev. Drug Discov. 12, 526–542 (2013).
Rose, P. G. Chemoradiotherapy for cervical cancer. Eur. J. Cancer 38, 270–278 (2002).
Eifel, P. J. Concurrent chemotherapy and radiation therapy as the standard of care for cervical cancer. Nat. Clin. Pract. Oncol. 3, 248–255 (2006).
Cheetham, P. & Petrylak, D. P. Tubulin-targeted agents including docetaxel and cabazitaxel. Cancer J. 19, 59–65 (2013).
Hennequin, C., Giocanti, N. & Favaudon, V. Interaction of ionizing radiation with paclitaxel (Taxol) and docetaxel (Taxotere) in HeLa and SQ20B cells. Cancer Res. 56, 1842–1850 (1996).
Mason K. A. et al. Effect of docetaxel on the therapeutic ratio of fractionated radiotherapy in vivo. Clin. Cancer Res. 5, 4191–4198 (1999).
Bolla, M. et al. Concurrent and adjuvant docetaxel with three-dimensional conformal radiation therapy plus androgen deprivation for high-risk prostate cancer: preliminary results of a multicentre phase II trial. Radiother. Oncol. 97, 312–317 (2010).
Swanson, G. P. et al. Locally advanced prostate cancer treated with concomitant radiation and 5-fluorouracil: Southwest Oncology Group Study 9024. J. Urol. 176, 548–553 (2006).
Perrotti, M. et al. Phase I/II trial of docetaxel and concurrent radiation therapy in localized high risk prostate cancer (AGUSG 03-10). Urol. Oncol. 26, 276–280 (2008).
Chen, R. C. et al. Phase I study of concurrent weekly docetaxel, high-dose intensity-modulated radiation therapy (IMRT) and androgen-deprivation therapy (ADT) for high-risk prostate cancer. BJU Int. 110, E721–E726 (2012).
Marshall, D. T. et al. Phase I trial of weekly docetaxel, total androgen blockade, and image-guided intensity-modulated radiotherapy for localized high-risk prostate adenocarcinoma. Clin. Genitourin. Cancer 12, 80–86 (2014).
Sanfilippo, N. J., Taneja, S. S., Chachoua, A., Lepor, H. & Formenti, S. C. Phase I/II study of biweekly paclitaxel and radiation in androgen-ablated locally advanced prostate cancer. J. Clin. Oncol. 26, 2973–2978 (2008).
Kumar, P. et al. Phase I trial of weekly docetaxel with concurrent three-dimensional conformal radiation therapy in the treatment of unfavorable localized adenocarcinoma of the prostate. J. Clin. Oncol. 22, 1909–1915 (2004).
Ben-Josef, E. et al. Neoadjuvant estramustine and etoposide followed by concurrent estramustine and definitive radiotherapy for locally advanced prostate cancer: feasibility and preliminary results. Int. J. Radiat. Oncol. Biol. Phys. 49, 699–703 (2001).
Zelefsky, M. J. et al. Results of a phase II study using estramustine phosphate and vinblastine in combination with high-dose three-dimensional conformal radiotherapy for patients with locally advanced prostate cancer. J. Clin. Oncol. 18, 1936–1941 (2000).
Khil, M. S., Kim, J. H., Bricker, L. J. & Cerny, J. C. Tumor control of locally advanced prostate cancer following combined estramustine, vinblastine, and radiation therapy. Cancer J. Sci. Am. 3, 289–296 (1997).
Makkouk, A. & Weiner, G. J. Cancer immunotherapy and breaking immune tolerance: new approaches to an old challenge. Cancer Res. 75, 5–10 (2015).
Ko, E. C. & Formenti, S. C. Radiotherapy and checkpoint inhibitors: a winning new combination? Ther. Adv. Med. Oncol. 10, 1758835918768240 (2018).
Finkelstein, S. E. et al. Combining immunotherapy and radiation for prostate cancer. Clin. Genitourin. Cancer 13, 1–9 (2015).
Belderbos, R. A., Aerts, J. G. J. V. & Vroman, H. Enhancing dendritic cell therapy in solid tumors with immunomodulating conventional treatment. Mol. Ther. Oncolytics 13, 67–81 (2019).
Schoenhals, J. E. et al. Optimizing radiotherapy with immunotherapeutic approaches. Adv. Exp. Med. Biol. 995, 53–71 (2017).
Vatner, R. E., Cooper, B. T., Vanpouille-Box, C., Demaria, S. & Formenti, S. C. Combinations of immunotherapy and radiation in cancer therapy. Front. Oncol. 4, 325 (2014).
Liechtenstein, T., Dufait, I., Lanna, A., Breckpot, K. & Escors, D. Modulating co-stimulation during antigen presentation to enhance cancer immunotherapy. Immunol. Endocr. Metab. Agents Med. Chem. 12, 224–235 (2012).
Kwilas, A. R., Donahue, R. N., Bernstein, M. B. & Hodge, J. W. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front. Oncol. 2, 104 (2012).
Panaretakis, T. et al. Mechanisms of pre-apoptotic calreticulin exposure in immunogenic cell death. EMBO J. 28, 578–590 (2009).
Wang, Y. et al. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front. Pharmacol. 9, 185 (2018).
Yin, Z., Li, C., Wang, J. & Xue, L. Myeloid-derived suppressor cells: roles in the tumor microenvironment and tumor radiotherapy. Int. J. Cancer. 144, 933–946 (2019).
Kumari, A., Simon, S. S., Moody, T. D. & Garnett-Benson, C. Immunomodulatory effects of radiation: what is next for cancer therapy? Future Oncol. 12, 239–256 (2016).
Reits, E. A. et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 203, 1259–1271 (2006).
Bernstein, M. B. et al. Radiation-induced modulation of costimulatory and coinhibitory T-cell signaling molecules on human prostate carcinoma cells promotes productive antitumor immune interactions. Cancer Biother. Radiopharm. 29, 153–161 (2014).
Garnett, C. T. et al. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 64, 7985–7994 (2004).
Aryankalayil, M. J. et al. Defining molecular signature of pro-immunogenic radiotherapy targets in human prostate cancer cells. Radiat. Res. 182, 139–148 (2014).
Hallahan, D. E., Spriggs, D. R., Beckett, M. A., Kufe, D. W. & Weichselbaum, R. R. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc. Natl Acad. Sci. USA 86, 10104–10107 (1989).
Rini, B. I. Technology evaluation: APC-8015, Dendreon. Curr. Opin. Mol. Ther. 4, 76–79 (2002).
Madan, R. A., Arlen, P. M., Mohebtash, M., Hodge, J. W. & Gulley, J. L. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert. Opin. Investig. Drugs 18, 1001–1011 (2009).
Burotto, M., Singh, N., Heery, C. R., Gulley, J. L. & Madan, R. A. Exploiting synergy: immune-based combinations in the treatment of prostate cancer. Front. Oncol. 4, 351 (2014).
Bilusic, M., Madan, R. A. & Gulley, J. L. Immunotherapy of prostate cancer: facts and hopes. Clin. Cancer Res. 23, 6764–6770 (2017).
Finkelstein, S. E. et al. Serial assessment of lymphocytes and apoptosis in the prostate during coordinated intraprostatic dendritic cell injection and radiotherapy. Immunotherapy 4, 373–382 (2012).
Egen, J. G., Kuhns, M. S. & Allison, J. P. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nat. Immunol. 3, 611–618 (2002).
Dewan, M. Z. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Slovin, S. F. et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann. Oncol. 24, 1813–1821 (2013).
Kwon, E. D. et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 700–712 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00861614 (2016).
Keir, M. E., Butte, M. J., Freeman, G. J. & Sharpe, A. H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008).
Gong, J., Le, T. Q., Massarelli, E., Hendifar, A. E. & Tuli, R. Radiation therapy and PD-1/PD-L1 blockade: the clinical development of an evolving anticancer combination. J. Immunother. Cancer 6, 46 (2018).
Dudzinski, S. O. et al. Combination immunotherapy and radiotherapy causes an abscopal treatment response in a mouse model of castration resistant prostate cancer. J. Immunother. Cancer 7, 218 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03543189 (2019).
Lesueur, P. et al. Poly-(ADP-ribose)-polymerase inhibitors as radiosensitizers: a systematic review of pre-clinical and clinical human studies. Oncotarget 8, 69105–69124 (2017).
Taylor, R. A. et al. The influence of BRCA2 mutation on localized prostate cancer. Nat. Rev. Urol. 16, 281–290 (2019).
Turk, A. A. & Wisinski, K. B. PARP inhibitors in breast cancer: bringing synthetic lethality to the bedside. Cancer 124, 2498–2506 (2018).
Virtanen, V. et al. PARP inhibitors in prostate cancer — the preclinical rationale and current clinical development. Genes 10, E565 (2019).
Keung, M., Wu, Y. & Vadgama, J. PARP inhibitors as a therapeutic agent for homologous recombination deficiency in breast cancers. J. Clin. Med. 8, E435 (2019).
Gui, B. et al. Selective targeting of PARP-2 inhibits androgen receptor signaling and prostate cancer growth through disruption of FOXA1 function. Proc. Natl Acad. Sci. USA 116, 14573–14582 (2019).
Shimizu, S. et al. Expression of poly(ADP-ribose) polymerase in human hepatocellular carcinoma and analysis of biopsy specimens obtained under sonographic guidance. Oncol. Rep. 12, 821–825 (2004).
Rojo, F. et al. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann. Oncol. 23, 1156–1164 (2012).
Domagala, P., Huzarski, T., Lubinski, J., Gugala, K. & Domagala, W. PARP-1 expression in breast cancer including BRCA1-associated, triple negative and basal-like tumors: possible implications for PARP-1 inhibitor therapy. Breast Cancer Res. Treat. 127, 861–869 (2011).
Lee, H.-J. et al. Combining PARP-1 inhibition and radiation in Ewing sarcoma results in lethal DNA damage. Mol. Cancer Ther. 12, 2591–2600 (2013).
Lemasson, B. et al. Evaluation of concurrent radiation, temozolomide and ABT-888 treatment followed by maintenance therapy with temozolomide and ABT-888 in a genetically engineered glioblastoma mouse model. Neoplasia. 18, 82–89 (2016).
Mateo, J. et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 373, 1697–1708 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03076203 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03317392 (2020).
Chang, L. et al. PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis. 5, e1437 (2014).
Jamaspishvili, T. et al. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 15, 222–234 (2018).
Potiron, V. A. et al. Radiosensitization of prostate cancer cells by the dual PI3K/mTOR inhibitor BEZ235 under normoxic and hypoxic conditions. Radiother. Oncol. 106, 138–146 (2013).
Chang, L. et al. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 4, e875 (2013).
Rudner, J. et al. The Akt-inhibitor Erufosine induces apoptotic cell death in prostate cancer cells and increases the short term effects of ionizing radiation. Radiat. Oncol. 5, 108 (2010).
Gao, Y. et al. The alkylphospholipid, perifosine, radiosensitizes prostate cancer cells both in vitro and in vivo. Radiat. Oncol. 6, 39 (2011).
Diaz, R. et al. The novel Akt inhibitor Palomid 529 (P529) enhances the effect of radiotherapy in prostate cancer. Br. J. Cancer 100, 932–940 (2009).
Dumont, R. A. et al. Targeted radiotherapy of prostate cancer with a gastrin-releasing peptide receptor antagonist is effective as monotherapy and in combination with rapamycin. J. Nucl. Med. 54, 762–769 (2013).
Schiewer, M. J. et al. mTOR is a selective effector of the radiation therapy response in androgen receptor-positive prostate cancer. Endocr. Relat. Cancer 19, 1–12 (2012).
Cao, C. et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 66, 10040–10047 (2006).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00943956 (2016).
Azria, D. et al. Concurrent treatment with everolimus (RAD001) and hormonoradiotherapy in high-risk locally advanced prostate cancer: results of a phase I trial. J. Clin. Oncol. 31, 150–150 (2013).
Rowlands, M. A. et al. Circulating insulin-like growth factors and IGF-binding proteins in PSA-detected prostate cancer: the large case-control study ProtecT. Cancer Res. 72, 503–515 (2012).
Alcorn, S. et al. Molecularly targeted agents as radiosensitizers in cancer therapy — focus on prostate cancer. Int. J. Mol. Sci. 14, 14800–14832 (2013).
Abrams, T. J., Lee, L. B., Murray, L. J., Pryer, N. K. & Cherrington, J. M. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol. Cancer Ther. 2, 471–478 (2003).
Abrams, T. J. et al. Preclinical evaluation of the tyrosine kinase inhibitor SU11248 as a single agent and in combination with "standard of care" therapeutic agents for the treatment of breast cancer. Mol. Cancer Ther. 2, 1011–1021 (2003).
Mendel, D. B. et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9, 327–337 (2003).
O’Farrell, A.-M. et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 101, 3597–3605 (2003).
Schueneman, A. J. et al. SU11248 maintenance therapy prevents tumor regrowth after fractionated irradiation of murine tumor models. Cancer Res. 63, 4009–4016 (2003).
Montero, J. C., Seoane, S., Ocaña, A. & Pandiella, A. Inhibition of SRC family kinases and receptor tyrosine kinases by dasatinib: possible combinations in solid tumors. Clin. Cancer Res. 17, 5546–5552 (2011).
Rice, L., Lepler, S., Pampo, C. & Siemann, D. W. Impact of the SRC inhibitor dasatinib on the metastatic phenotype of human prostate cancer cells. Clin. Exp. Metastasis. 29, 133–142 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01826838 (2015).
Barrott, J. J. & Haystead, T. A. J. Hsp90, an unlikely ally in the war on cancer. FEBS J. 280, 1381–1396 (2013).
Georget, V., Térouanne, B., Nicolas, J.-C. & Sultan, C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry 41, 11824–11831 (2002).
Boevé, L. M. S. et al. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial. Eur. Urol. 75, 410–418 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT03678025 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT01957436 (2019).
Moghanaki, D. et al. Advances in prostate cancer magnetic resonance imaging and positron emission tomography-computed tomography for staging and radiotherapy treatment planning. Semin. Radiat. Oncol. 27, 21–33 (2017).
Palacios-Eito, A., Béjar-Luque, A., Rodríguez-Liñán, M. & García-Cabezas, S. Oligometastases in prostate cancer: ablative treatment. World J. Clin. Oncol. 10, 38–51 (2019).
O’Shaughnessy, M. J. et al. A pilot study of a multimodal treatment paradigm to accelerate drug evaluations in early-stage metastatic prostate cancer. Urology 102, 164–172 (2017).
Glehen, O., Mohamed, F. & Gilly, F. N. Peritoneal carcinomatosis from digestive tract cancer: new management by cytoreductive surgery and intraperitoneal chemohyperthermia. Lancet Oncol. 5, 219–228 (2004).
Temple, L. K. F., Hsieh, L., Wong, W. D., Saltz, L. & Schrag, D. Use of surgery among elderly patients with stage IV colorectal cancer. J. Clin. Oncol. 22, 3475–3484 (2004).
Bristow, R. E., Tomacruz, R. S., Armstrong, D. K., Trimble, E. L. & Montz, F. J. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J. Clin. Oncol. 20, 1248–1259 (2002).
Mickisch, G. H., Garin, A., van Poppel, H., de Prijck, L. & Sylvester, R., European Organisation for Research and Treatment of Cancer (EORTC) Genitourinary Group. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 358, 966–970 (2001).
Muacevic, A. et al. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol. Oncol. 31, 455–460 (2013).
Ahmed, K. A. et al. Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer. Front. Oncol. 2, 215 (2012).
Berkovic, P. et al. Salvage stereotactic body radiotherapy for patients with limited prostate cancer metastases: deferring androgen deprivation therapy. Clin. Genitourin. Cancer 11, 27–32 (2013).
Schick, U. et al. Androgen deprivation and high-dose radiotherapy for oligometastatic prostate cancer patients with less than five regional and/or distant metastases. Acta Oncol. 52, 1622–1628 (2013).
Decaestecker, K. et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat. Oncol. 9, 135 (2014).
Ost, P. It ain’t over till the fat lady sings: the POPSTAR trial. Eur. Urol. 74, 463–464 (2018).
Siva, S. et al. Stereotactic abative body radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur. Urol. 74, 455–462 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02759783 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/results/NCT02685397 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03569241 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02680587 (2019).
Radwan, N. et al. A phase II randomized trial of OBSERVATION versus stereotactic ablative RadiatIon for OLigometastatic prostate CancEr (ORIOLE). BMC Cancer 17, 453 (2017).
Phillips R. M., Deek M. P., Deweese T. L. & Tran P. T. Metastasis-directed therapy in prostate cancer. Why, when, and how? Cancer Netw. 33, 686509 (2019).
Solberg, T. D. et al. Quality and safety considerations in stereotactic radiosurgery and stereotactic body radiation therapy: executive summary. Pract. Radiat. Oncol. 2, 2–9 (2012).
SABR UK Consortium. Stereotactic ablative body radiation therapy (SABR): a resource. SABR UK Consortium https://www.sabr.org.uk/wp-content/uploads/2019/04/SABRconsortium-guidelines-2019-v6.1.0.pdf (2019).
Kamran, S. C. & Mouw, K. W. Applying precision oncology principles in radiation oncology. JCO Precis. Oncol. 14, 1–23 (2018).
Berlin, A. et al. Genomic classifier for guiding treatment of intermediate-risk prostate cancers to dose-escalated image guided radiation therapy without hormone therapy. Int. J. Radiat. Oncol. Biol. Phys. 103, 84–91 (2019).
Yang, L. et al. Development and validation of a 28-gene hypoxia-related prognostic signature for localized prostate cancer. EBioMedicine. 31, 182–189 (2018).
Wang, J., Boerma, M., Fu, Q. & Hauer-Jensen, M. Significance of endothelial dysfunction in the pathogenesis of early and delayed radiation enteropathy. World J. Gastroenterol. 3, 3047–3055 (2007).
Heckmann, M., Douwes, K., Peter, R. & Degitz, K. Vascular activation of adhesion molecule mRNA and cell surface expression by ionizing radiation. Exp. Cell Res. 238, 148–154 (1998).
Baker, D. G. & Krochak, R. J. The response of the microvascular system to radiation: a review. Cancer Invest. 7, 287–294 (1989).
Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 4, 437–447 (2004).
Harris, A. L. Hypoxia — a key regulatory factor in tumour growth. Nat. Rev. Cancer 2, 38–47 (2002).
Horsman, M. R. & Overgaard, J. The impact of hypoxia and its modification of the outcome of radiotherapy. J. Radiat. Res. 57, i90–i98 (2016).
Bridges, E. M. & Harris, A. L. The angiogenic process as a therapeutic target in cancer. Biochem. Pharmacol. 81, 1183–1191 (2011).
Goel, S., Wong, A. H.-K. & Jain, R. K. Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb. Perspect. Med. 2, a006486 (2012).
Potiron, V. A. et al. Improved functionality of the vasculature during conventionally fractionated radiation therapy of prostate cancer. PLoS ONE 8, e84076 (2013).
Wu, J.-B., Tang, Y.-L. & Liang, X.-H. Targeting VEGF pathway to normalize the vasculature: an emerging insight in cancer therapy. Onco Targets Ther. 11, 6901–6909 (2018).
Jain, R. K. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med. 7, 987–989 (2001).
Carmeliet, P. & Jain, R. K. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat. Rev. Drug Discov. 10, 417–427 (2011).
Winkler, F. et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation. Cancer Cell. 6, 553–563 (2004).
Mauceri, H. J. et al. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature 394, 287–291 (1988).
Lee, C. G. et al. Anti-vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 60, 5565–5570 (2000).
Kozin, S. V. et al. Vascular endothelial growth factor receptor-2-blocking antibody potentiates radiation-induced long-term control of human tumor xenografts. Cancer Res. 61, 39–44 (2001).
Kanthou, C. & Tozer, G. Targeting the vasculature of tumours: combining VEGF pathway inhibitors with radiotherapy. Br. J. Radiol. 92, 20180405 (2019).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 21, 137–148 (2004).
Dunn, G. P., Old, L. J. & Schreiber, R. D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 22, 329–360 (2004).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Walle, T. et al. Radiation effects on antitumor immune responses: current perspectives and challenges. Ther. Adv. Med. Oncol. 10, 1758834017742575 (2018).
Locy, H. et al. Immunomodulation of the tumor microenvironment: turn foe into friend. Front. Immunol. 9, 2909 (2018).
Postow, M. A. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366, 925–931 (2012).
Kepp, O. et al. Molecular determinants of immunogenic cell death elicited by anticancer chemotherapy. Cancer Metastasis Rev. 30, 61–69 (2011).
Kroemer, G., Galluzzi, L., Kepp, O. & Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 31, 51–72 (2013).
Töpfer, K. et al. Tumor evasion from T cell surveillance. J. Biomed. Biotechnol. 2011, 918471 (2011).
Mocellin, S. & Nitti, D. Therapeutics targeting tumor immune escape: towards the development of new generation anticancer vaccines. Med. Res. Rev. 28, 413–444 (2008).
Bronte, V. & Mocellin, S. Suppressive influences in the immune response to cancer. J. Immunother. 32, 1–11 (2009).
Young, K. H. et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS ONE 11, e0157164 (2016).
Dovedi, S. J. et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 74, 5458–5468 (2014).
Lugade, A. A. et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J. Immunol. 174, 7516–7523 (2005).
Lee, Y. et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 114, 589–595 (2009).
Hanks, G. E. et al. Dose selection for prostate cancer patients based on dose comparison and dose response studies. Int. J. Radiat. Oncol. Biol. Phys. 46, 823–832 (2000).
Koontz, B. F., Bossi, A., Cozzarini, C., Wiegel, T. & D’Amico, A. A systematic review of hypofractionation for primary management of prostate cancer. Eur. Urol. 68, 683–691 (2015).
Zilli, T. et al. ONE SHOT-single shot radiotherapy for localized prostate cancer: study protocol of a single arm, multicenter phase I/II trial. Radiat. Oncol. 13, 166 (2018).
Tselis, N. et al. High dose rate brachytherapy as monotherapy for localised prostate cancer: review of the current status. Clin. Oncol. 29, 401–411 (2017).
Xu, M. J. et al. Single-fraction brachytherapy as monotherapy for early-stage prostate cancer: the UCSF experience. Brachytherapy 18, 470–476 (2019).
Hauswald, H. et al. High-dose-rate monotherapy for localized prostate cancer: 10-year results. Int. J. Radiat. Oncol. Biol. Phys. 94, 667–674 (2016).
Acknowledgements
Research in the laboratory of R.J.B. is funded by a Cancer Research UK/Royal College of Surgeons of England Clinician Scientist Fellowship (reference C39297/A22748), and research grants from the Urology Foundation (to Y.P.), and the Oxford-based charity Urology Cancer Research and Education (UCARE; to R.J.B.). Research in the laboratory of A.D.L. is funded by a Cancer Research UK Clinician Scientist Fellowship (reference C57899/A25812).
Reviewer information
Nature Reviews Urology thanks B. Koontz and the other, anonymous, reviewer(s) for their help with the peer review of this manuscript.
Author information
Authors and Affiliations
Contributions
Y.P. and R.J.B researched data for the article, made substantial contributions to discussions of content, and wrote the article. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- External beam radiotherapy
-
(EBRT). External delivery of irradiation using several types of energy (for example photons, electrons or heavy particles).
- Brachytherapy
-
Internalized radiotherapy using implanted iodine-125, palladium-103 or caesium-131 radioisotope seeds.
- Oligometastatic prostate cancer
-
Prostate cancer with relatively few (three to five) small-volume distant disease foci.
- 5Rs of radiation biology
-
Repair, reassortment, repopulation, reoxygenation and radiosensitivity. Each of these terms describes a cellular mechanism by which to understand the success or failure of localized radiotherapy.
- Mitotic catastrophe
-
A mechanism of delayed mitosis-linked cell death. It describes a sequence of events resulting from premature or inappropriate entry of cells into mitosis, caused by chemical or physical stresses.
- Immunogenic cell death
-
Immune-mediated cell killing secondary to radiation-induced generation of mutated tumour antigens, which subsequently stimulate the immune system.
- Stereotactic body radiotherapy
-
(SBRT). Delivery of external beam radiotherapy for treatment of prostate cancer, with target localization using image guidance and five to seven fractions of 6–10 Gy per fraction to the prostate gland.
- Bragg peak
-
The peak on the Bragg curve, which plots the energy loss of ionizing radiation (protons, α-rays and other ion rays) as they travel through matter. The peak occurs immediately before the particles come to rest.
- Salvage radiotherapy
-
Delivery of radiotherapy with curative intent to patients with biochemical recurrence (defined as a postoperative serum PSA level ≥0.2 ng/ml, without evidence of distant metastases).
- Immunomodulation
-
Regulation of the immune system, whereby the immune responses are induced, amplified, reduced or prevented according to the therapeutic goal.
- Tumour-associated antigen
-
An antigenic peptide produced in tumour cells that can trigger an immune response. It could potentially be used as a tumour marker and/or a therapeutic target.
- Calreticulin
-
An endoplasmic reticulum-associated chaperone protein, which is exposed in the outer leaflet of the plasma membrane of stressed or dying cells, where it functions as a potent phagocytosis signal.
- HMGB1
-
High mobility group box 1 protein is encoded by the HMGB1 gene and functions to organize DNA and regulate transcription.
- CTLA4
-
Cytotoxic T lymphocyte associated protein 4 is a protein receptor that functions as an immune checkpoint and downregulates immune responses.
- Abscopal effect
-
A systemic antitumour immune response induced by local irradiation, with regression of non-irradiated metastatic lesions at a distance from the primary site of irradiation.
- PD1
-
Programmed cell death protein 1 is a cell surface protein that downregulates the immune system’s response by suppressing T cell inflammatory activity.
- PDL1
-
Programmed cell death 1 ligand 1 is a trans-membrane protein that suppresses the adaptive immune system.
- FAK
-
Focal adhesion kinase is a protein involved in cell–cell adhesion and migration, and which can promote malignant cellular invasion and metastasis.
- HSP90
-
Heat shock protein 90 is a chaperone protein that promotes protein folding and stabilization against heat stress, thereby potentially promoting tumour growth.
- HIF1α
-
Hypoxia-inducible factor 1α is a subunit of the heterodimeric hypoxia-inducible factor 1 (HIF1) transcription factor encoded by the HIF1A gene.
- VEGFA
-
Vascular endothelial growth factor A is a peptide encoded by the VEGFA gene and acts on endothelial cells to increase vascular permeability, induce angiogenesis, vasculogenesis and endothelial cell growth, to promote cellular migration and to inhibit apoptosis.
- VEGFR1
-
Vascular endothelial growth factor receptor 1 is a protein with tyrosine protein kinase activity that regulates cellular proliferation and differentiation.
- Tumour vessel normalization
-
A spectrum of biological changes to the tumour vasculature including increased pericyte coverage, improved perfusion, reduced vascular permeability and reduced hypoxia.
Rights and permissions
About this article
Cite this article
Philippou, Y., Sjoberg, H., Lamb, A.D. et al. Harnessing the potential of multimodal radiotherapy in prostate cancer. Nat Rev Urol 17, 321–338 (2020). https://doi.org/10.1038/s41585-020-0310-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-020-0310-3
This article is cited by
-
Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy
Signal Transduction and Targeted Therapy (2022)
-
Synergistic effects of radiotherapy and targeted immunotherapy in improving tumor treatment efficacy: a review
Clinical and Translational Oncology (2022)
-
Cell death in pancreatic cancer: from pathogenesis to therapy
Nature Reviews Gastroenterology & Hepatology (2021)
-
Tumour irradiation combined with vascular-targeted photodynamic therapy enhances antitumour effects in pre-clinical prostate cancer
British Journal of Cancer (2021)