Abstract
Conventional photosystem II (PSII) herbicides applied in agriculture can pose significant environmental risks to aquatic environments. In response to the frequent detection of these herbicides in the Great Barrier Reef (GBR) catchment area, transitions towards ‘alternative’ herbicides are now widely supported. However, water quality guideline values (WQGVs) for alternative herbicides are lacking and their potential ecological impacts on tropical marine species are generally unknown. To improve our understanding of the risks posed by some of these alternative herbicides on marine species under tropical conditions, we tested the effects of four herbicides on the widely distributed diatom Chaetoceros muelleri. The PSII herbicides diuron, propazine, and tebuthiuron induced substantial reductions in both 24 h effective quantum yields (ΔF/Fm′) and 3-day specific growth rates (SGR). The effect concentrations, which reduced ΔF/Fm′ by 50% (EC50), ranged from 4.25 µg L−1 diuron to 48.6 µg L−1 propazine, while the EC50s for SGR were on average threefold higher, ranging from 12.4 µg L−1 diuron to 187 µg L−1 tebuthiuron. Our results clearly demonstrated that inhibition of ΔF/Fm′ in PSII is directly linked to reduced growth (R2 = 0.95) in this species, further supporting application of ΔF/Fm′ inhibition as a valid bioindicator of ecological relevance for PSII herbicides that could contribute to deriving future WQGVs. In contrast, SGR and ΔF/Fm′ of C. muelleri were nonresponsive to the non-PSII herbicide haloxyfop at the highest concentration tested (4570 µg L−1), suggesting haloxyfop does not pose a risk to C. muelleri. The toxicity thresholds (e.g. no effect concentrations; NECs) identified in this study will contribute to the derivation of high-reliability marine WQGVs for some alternative herbicides detected in GBR waters and support future assessments of the cumulative risks of complex herbicide mixtures commonly detected in coastal waters.
Similar content being viewed by others
Introduction
Herbicide contamination in the Great Barrier Reef
Herbicide contamination of nearshore waters is common across tropical regions, including the Caribbean1, Mexico2, Central America3 and the Asia-Pacific4,5,6,7,8; however, the Great Barrier Reef (GBR) World Heritage Area located on the east coast of northern Queensland, Australia represents the most studied location for herbicide contamination in tropical waters9. The GBR is the world’s largest reef ecosystem, containing extensive areas of seagrass meadows, mangroves, and coral reefs10. The GBR catchment (> 400,000 km2) accommodates a large agricultural industry that comprises of row crops (mainly sugarcane cultivation and horticulture) and cattle grazing in which pesticides are commonly applied to control weeds and other pests9,11,12. With > 35 major rivers discharging into the GBR lagoon, pesticide contamination from nearshore agricultural runoff is recognized as one of several threats faced by tropical nearshore ecosystems that need to be managed to maintain the health of this ecosystem13. Pesticides detected in waters of the GBR include herbicides, insecticides and fungicides, with long-term water quality monitoring programs most frequently detecting a group of five photosystem II (PSII) herbicides (diuron, ametryn, atrazine, tebuthiuron, and hexazinone)9,14,15,16,17. PSII herbicides are designed to target weeds by competing with plastoquinone for the secondary quinone QB binding site on the D1 protein within the thylakoid membrane18. This results in the interruption of the electron transport from the primary quinone QA to QB and subsequently, light-induced degradation of the D1 protein and reduced photochemical energy conversion within PSII18. Since all plants rely on the function of PSII, these herbicides can be equally effective at harming non-target species as the weeds they were designed to control. Due to their widespread application in agricultural industries, these herbicides are found throughout the nearshore waters of the GBR, but more frequently and at higher concentrations following riverine flood events (December to April)12,19,20,21 in which peak concentrations of up to 22 µg L−1 diuron have been detected in grab samples flowing into the GBR lagoon12. However, their continuous application and persistence22,23 contributes to year-round detections15,24. Consequently, these herbicides are considered ‘priority’ herbicides for management action designed to reduce the potential impacts of contaminants in waters of the GBR and its catchments13. To help achieve targeted reductions in priority herbicide loads, ’alternative’ PSII herbicides and ‘alternative’ non-PSII herbicides are increasingly applied as substitutes for effective weed control25. At present, sixteen alternative herbicides with five modes of action have been detected in GBR waters in addition to the priority PSII herbicides24. Alternative herbicides can exhibit similarities in physico-chemical properties to the priority PSII herbicides and in some cases, can be just as toxic to non-target marine species26. Nevertheless, their potential ecological impacts, particularly of non-PSII herbicides, on aquatic environments are generally unknown9.
Improving water quality guideline values for pesticides
The risks posed to aquatic habitats by contaminants are generally assessed by comparing measured concentrations in the field against water quality guideline values (WQGVs). In Australia, national WQGVs (referred to by the Australian and New Zealand Guidelines for Fresh and Marine Water Quality (ANZG)27 as default GVs) are derived to protect 99%, 95%, 90% and 80% (PC99, 95, 90, 80, respectively) of marine and freshwater species by assessing community sensitivities from species sensitivity distributions (SSDs)28. These SSDs are derived from toxicity threshold data of at least five species from at least four phyla that are representative of the receiving environment28. Currently, the ANZG include marine WQGVs for only four alternative herbicides: bromacil, simazine, 2,4-D, and MCPA, and the priority PSII herbicides (except for ametryn)27. However, with exception from diuron, these marine WQGVs are of low reliability as they were adapted from freshwater toxicity thresholds27.
Regular monitoring of pesticides in the GBR has found that exceedances of WQGVs by individual herbicides in the GBR marine waters occur only occasionally15,16,24; however, approximately 80% of the water samples collected in the GBR catchment area between 2011 and 2015 contained mixtures of up to 20 pesticides with two to four modes of action29. Consequently, there is a strong likelihood of additivity or synergistic interactions between multiple herbicides, and the total toxicity of herbicide mixtures should be considered in monitoring programs and for risk assessments14,30,31,32. To predict the cumulative risk of herbicide mixtures, a more comprehensive risk assessment approach has been proposed which applies the multisubstance-potentially affected fraction (ms-PAF) method33. In cases where the combined concentrations of multiple co-occurring herbicides are considered using the ms-PAF approach, WQGV exceedances in the GBR become more frequent24. The ms-PAF method has also recently been extended to adjust herbicide WQGVs for heatwave conditions often faced by tropical marine species34. Improved WQGVs for alternative herbicides are therefore required so that the ms-PAF method can take into consideration all herbicides detected in water samples for assessing the total risk. A revision of the current WQGVs, including 13 alternative herbicides, has been proposed based on all available marine and freshwater toxicity data35,36. Nevertheless, most of the proposed guideline values (PGVs) are still of low reliability and many data gaps remain, especially for marine species. Consequently, additional toxicity testing of most herbicides using marine phototrophs is recommended for improving their reliability35,36.
Toxicity testing with marine microalgae
Marine microalgae form an essential functional group as primary producers. However, herbicide-induced damage to PSII leads to declining growth rates and biomass of microalgae and consequently, may initiate indirect bottom-up effects on higher trophic levels due to changes in their community structure37. Non-PSII herbicides can also affect microalga, but their vulnerability depends on whether the mode of action of the herbicide is also relevant to each specific type of algae. Their ecological importance, potential vulnerability to herbicides, along with rapid growth rates that allow for chronic exposure testing in a short period, mean that marine microalgae represent a suitable taxon to contribute to improving WQGVs. Currently, SSDs used to derive high-quality WQGVs require ecologically relevant toxicity data, and for microalgae, the inhibition of growth is the most common endpoint27,28. Another more rapid and sensitive technique to quantify the toxicity of PSII herbicides to marine phototrophs takes advantage of increased chlorophyll a fluorescence emissions that result from the excess excitation energy that would normally drive electron transport in PSII but is blocked by these herbicides38. This results in reduced photosynthetic efficiency (effective quantum yield: ΔF/Fm′) which can be measured by pulse amplitude modulation (PAM) fluorometry39. PAM fluorometry has been extensively applied for assessing sub-lethal effects of PSII herbicides in microalgae40,41,42,43; however, several studies have demonstrated that this method can be far less sensitive to non-PSII herbicides, where the mode of action does not involve PSII26,44,45. Nevertheless, further assessment of ΔF/Fm′ inhibition as an effective endpoint for herbicides is warranted to investigate its suitability as an ecologically relevant endpoint to support herbicide risk assessments.
In order to improve WQGVs for herbicides detected in GBR waters, more toxicity data is required for deriving high-quality SSDs. Here, we tested the individual effects of four herbicides on the growth and ΔF/Fm′ of the diatom Chaetoceros muelleri, which was selected as a representative of the phylum Bacillariophyta, generally underrepresented in current SSDs. Additionally, this study aimed to estimate no effect concentrations (NECs) for single herbicides which are the preferred toxicity thresholds for inclusion in SSDs to derive WQGVs28. Based on consultation with the Water Quality and Investigation Team at the Queensland Department of Environment and Science (DES) three herbicides that indicated current data gaps were chosen for testing, along with the reference PSII herbicide diuron. The tested herbicides included the PSII herbicides tebuthiuron and propazine, as well as the non-PSII herbicide haloxyfop. The toxicity thresholds identified here provide valuable toxicity data for some PSII and a non-PSII herbicide detected in GBR waters to contribute to improved WQGVs that are necessary for adequate protection of marine species and application in risk assessments.
Results
Toxicity test performance
Control growth rates of the test species C. muelleri were consistently > 1 doublings day-1 across all four 3-day experiments with SGR ranging from 1.41 ± 0.05 day−1 to 1.68 ± 0.05 day−1 (mean ± SD) (Table 1). The percent coefficient of variation (% CV) for each test was ≤ 5%, indicating test acceptability46 for all toxicity tests (Table 1). Chlorophyll fluorescence control measurements were also consistent over the exposure period of 24 h, with ΔF/Fm′ control measurements across all tests varying between 0.418 ± 0.015 and 0.478 ± 0.005 (mean ± SD). The ethanol carrier solvent (< 0.01% v/v) had no significant influence on SGR compared with filtered seawater (FSW) after 3-days (ANOVA, Fethanol (1,3) = 3.23, p = 0.17). The effect of the reference toxicant diuron applied at 4 µg L−1 across all experiments also inhibited SGR and ΔF/Fm′ consistently across all tests (Table 1). Physicochemical measurements of salinity (33.3–35.3 psu; range across all herbicide tests), dissolved oxygen (8.0–8.4 mg L−1), and temperature (26.9–27.8 °C) indicated little variation within each treatment and across all tests (Table S-3). Changes in pH across all tests varied between 8.0 and 8.5 over 3-days and remained within the acceptable range of < 1 pH unit change for each test (Table S-3)46. Nominal and measured concentrations of each herbicide are presented in Table S-2.
Toxicity of herbicides to microalgae
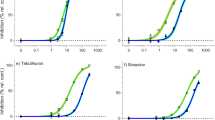
Concentration-dependent inhibition of growth and photosynthetic activity (ΔF/Fm′) was observed for all PSII herbicides tested (Fig. 1). These herbicides exhibited a broad range of potencies with diuron being the most toxic, inhibiting 50% of SGR and ΔF/Fm′ (EC50) at 12.4 µg L−1 and 4.25 µg L−1, respectively (Table 2). A comparison of relative potencies (ReP, based on EC50 values) against the reference herbicide diuron revealed that the least potent PSII herbicide to SGR was tebuthiuron (ReP = 0.066), indicating 15-times lower toxicity than diuron (Rep = 1) (Table 2). Based on ReP values for ΔF/Fm′ inhibition, the PSII herbicide propazine (ReP = 0.087) was least toxic to C. muelleri (Table 2) and was 11-fold less toxic than diuron (Table 2). The concentration–response curves all exhibited similar shapes and slopes (Fig. 1) with R2 values ≥ 0.98. The EC10 and predicted NEC values (from Figs. 1 and 2, respectively) were also reported in Table 2 and showed similar orders of toxicity. In contrast to the PSII herbicides, SGR and ΔF/Fm′ of C. muelleri were not affected by the acetyl-CoA carboxylase (ACCase) inhibitor haloxyfop at the maximum concentration of 4570 µg L−1 tested and no significant differences between treatments by ANOVA (F (6,28) = 2.2, p = 0.07; F (5,28) = 1.5, p = 0.24, respectively) were detected (Fig. 1). Higher concentrations were not tested due to its low water solubility47.
Concentration–response curves for ECx derivation. Sigmoidal, 4-parameter curve fit and 95% confidence intervals (shaded area) on the relative percent inhibition of 3-day specific growth rate (SGR) and 24 h effective quantum yield (ΔF/Fm′) of Chaetoceros muelleri (mean ± SD) following herbicide exposure to (a) Diuron; (b) Propazine; (c) Tebuthiuron and boxplot showing inhibition of 3-day specific growth rate (SGR) and effective quantum yield (ΔF/Fm′) in response to (d) Haloxyfop. All concentrations in µg L−1 (n = 5 for each treatment, error bars not visible are smaller than symbol).
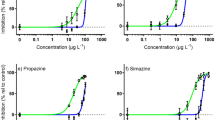
Concentration–response curves for NEC derivation. Bayesian non-linear gaussian model fit on the proportional decline in 3-day specific growth rate (SGR) relative to the control treatment (solid black line) and 95% confidence intervals (black dashed line) to derive the no effect concentration (NEC) (red line) and 95% confidence interval (red dashed line) of (a) Diuron; (b) Propazine; (c) Tebuthiuron. All concentrations in µg L−1.
Relationship between inhibition of effective quantum yield and growth
A comparison of ΔF/Fm′ and SGR inhibition due to PSII herbicides demonstrated that inhibition of ΔF/Fm′ was a more sensitive endpoint than inhibition of SGR (Fig. 3, Table 3). The regression analyses indicated linear relationships between response types for all three PSII herbicides with slopes that were close to unity (Table 3). However, the comparison of the EC50 ratios for SGR : ΔF/Fm′, which ranged from 2.0 to 3.9 (Table 2), revealed that inhibition in ΔF/Fm′ was on average a threefold more sensitive endpoint than inhibition in growth.
Discussion
Toxicity of PSII herbicides to microalgae
The three PSII herbicides induced substantial reductions in both ΔF/Fm′ and SGR of C. muelleri at relatively low concentrations (Table 2). PSII herbicides exert their toxicity by inhibiting the electron transport in the PSII complex, resulting in both reduced production and damage to the PSII system due to light-induced oxidative stress caused by formation of reactive oxygen species in the reaction center itself48. Prolonged exposure to reactive oxygen species can cause irreversible cell damage ultimately leading to cell death49. The inhibition of ΔF/Fm′ in C. muelleri by PSII herbicides can indicate both reduced photosynthetic efficiency caused by blockage of electron transport driving production and damage to PSII and both mechanisms are likely to have contributed to the inhibition in SGR. Based on the EC50s in this study, the phenylurea herbicide diuron was the most toxic herbicide towards C. muelleri, while the triazine herbicide propazine and the phenylurea herbicide tebuthiuron were 8–15-times less toxic to ΔF/Fm′ and SGR of C. muelleri (Table 2). Although PSII herbicides share the same mode of action toxicities of these herbicides significantly differed even for herbicides within the same chemical class, as demonstrated here for the phenylurea herbicides. Toxicity differences between herbicides of the same mode of action are consistent with other reports for marine microalgae26,30. The physico-chemical properties of the herbicides differ (Table S-1) but there was no clear direct relationship between these properties (KOW, water solubility etc.) and their toxicities. It is likely that herbicides that have greater affinities to the QB binding site and faster binding rates have greater toxic potential50.
Diuron is the most widely studied PSII herbicide with respect to tropical marine species, including corals51, foraminifera52, and macroalgae53 and inhibition of ΔF/Fm′ is the most commonly reported toxic endpoint. However, ecologically relevant endpoints related to mortality, reproductive effects and growth inhibition are required for WQGV derivation28, and growth inhibition is more often reported for marine microalgae. For example, 3–7-day SGR inhibition EC50 values range between 0.55–110 μg L−1 diuron across 27 marine microalgal species (Table 4)35,54. The 3-day SGR EC50 value derived here for C. muelleri places this species among the more sensitive of microalgal species in this dataset, but direct comparisons among toxicity tests should be made with caution due to differences in experimental conditions. However, direct comparisons of EC50s against the equivalent thresholds of the marine cryptophyte Rhodomonas salina is valid as this species was tested in the same laboratory under identical test conditions26. This comparison revealed that C. muelleri was twofold less sensitive to diuron. The toxic effects of the alternative PSII herbicide propazine on marine microalgae have been investigated in only two other studies. The SGR EC50 value for C. muelleri (Table 4) indicated this species was around fourfold less sensitive than the diatom Skeletonema costatum (Table 4), but unlike diuron twice as sensitive to propazine compared to R. salina (Table 4). Although tebuthiuron is considered a priority herbicide, little data has been published on its toxic effects on marine microalgae. Indeed, chronic toxicity values (EC50) were only reported for the marine diatom S. costatum (Table 4) and R. salina which was almost 2-times more sensitive (Table 4) than the reported EC50 value for C. muelleri (Table 4).
Differential responses of microalgae to PSII herbicides may partially be due to related differences in the molecular architecture of the D1 protein, as well as different mechanisms of photosynthetic acclimation to light63. For example, chlorophytes are often reported to be more susceptible to herbicides compared to ochrophytes due to differences in light-harvesting pigments and adaptation of ochrophytes to low light conditions64,65,66. Compared to chlorophytes, diatoms such as C. muelleri may apply an extra carbon fixation pathway, for example β-carboxylation that could compensate for herbicide-induced reduction in PSII-based photosynthesis, allowing some metabolism to continue67,68. Community changes of microalgae in response to chronic PSII herbicide exposure have been observed in several studies. For example, pollution-induced community tolerance in tropical estuarine periphyton in response to chronic diuron exposures was observed by Magnusson et al.69, leading to a shift in species composition towards communities dominated by diatoms. The mechanisms leading to community tolerance in microalgae were not fully investigated but may be related to the ability in some diatom species to switch to heterotrophic nutrient acquisition under these conditions40,70. These clear but often unpredictable differences in responses to herbicide exposure between alga stresses the importance of incorporating microalgae species from different taxa when deriving SSDs and WQGVs for environmental protection purposes.
Ecological risk of PSII herbicides
There are current marine WQGVs for diuron and tebuthiuron, but not for propazine in marine or freshwater environments27. Updated guideline values have recently been proposed (PGVs) for all three herbicides, however there were not enough data for marine phototrophs available to develop WQGVs for tebuthiuron and propazine, and therefore were derived from SSDs based on toxicity thresholds from both marine and freshwater taxa35,36. In fact, only two of seven species in the tebuthiuron SSD were marine and one of five species in the propazine SSD, highlighting the lack of tropical marine toxicity data for these herbicides. Consequently, the modeled distribution of these data resulted in low- to moderate-reliability PGVs that may not represent adequate protection to marine microalgae. Very high-reliability PGVs were able to be derived only for diuron as sufficient chronic toxicity data for marine phototrophs (in total 20 species) were available35.
Diuron, propazine, and tebuthiuron are all approved and registered for use in agricultural industries in the GBR catchment area71 but are more tightly regulated in the US72 and tebuthiuron and propazine do not have regulatory approval within the European Union73. From the most recent water quality monitoring year (2017–2018), these herbicides were reported as among the most frequently detected and abundant herbicides in nearshore waters of the GBR24 with frequencies between 40–80% in fixed (long-term) monitoring sites using passive samplers24. Maximum concentrations of these herbicides (typically identified in the Mackay-Whitsunday region) ranged from < 5 ng L−1 tebuthiuron and propazine to 778 ng L−1 diuron24. The concentration estimates from passive samplers can accurately estimate month-long averages, but concentrations of individual herbicides can reach over threefold higher concentrations during shorter duration pulses74. The 99% species protection (PC99) PGV of diuron (0.43 µg L−1), propazine (2.2 µg L−1), and tebuthiuron (4.7 µg L−1)35,36 were lower than the NEC values (1.47, 12.9 and 16 µg L−1, respectively) derived in this study, indicating that C. muelleri would be protected by the PGVs and are unlikely to be affected by most GBR field exposure concentrations of these herbicides individually. However, these highly mobile PSII herbicides47 have very long half-lives in marine waters75, contributing to their frequent year-round detection in complex mixtures24,29. It is therefore important that the risks posed by PSII herbicides should not be assessed individually. Instead, individual contributions to the risk posed by multi-herbicide mixtures should be assessed using ms-PAF33 which accounts for all herbicides that have reliable SSDs (and WQGVs). The individual toxicity thresholds (i.e. NECs) identified for C. muelleri here are intended to contribute to the future derivation of high-reliability marine WQGVs for the PSII herbicide diuron, propazine, and tebuthiuron and support assessments of cumulative risks of herbicide mixtures using ms-PAF.
Toxicity of non-PSII herbicides on microalgae
Haloxyfop belongs to the family of phenoxy herbicides and has been developed as a selective herbicide that is mainly absorbed through the foliage and roots of plants with subsequent hydrolysis to the acid, which is herbicidally active76. Haloxyfop inhibits the acetyl-CoA carboxylase (ACCase) enzyme that is involved in the synthesis of fatty acids76 and exists in two forms, the multi-subunit, prokaryotic (heteromeric) form and the multi-domain, eukaryotic (homomeric) form and in two locations (cytosol and plastid)77. Haloxyfop and other ACCase inhibitors target primarily the eukaryotic form of the enzyme rather than the prokaryotic form78. In the present study, both, SGR and ΔF/Fm′ of C. muelleri were nonresponsive to haloxyfop after 3-day exposure at the maximum concentration of 4570 µg L−1. In plants, both forms of ACCase enzyme have been described; however, some studies have indicated that certain microalgae, including some rhodophytes and chlorophytes, only contain the prokaryotic ACCase enzyme in their plastids77,79, possibly explaining the insensitivity of C. muelleri towards haloxyfop. There is only one other study on the toxicity of haloxyfop to marine phototrophs which reported a similar insensitivity in the marine cryptophyte Rhodomonas salina, with no inhibition of ΔF/Fm′ or SGR at the highest concentration of 3700 µg L−1 (Table 4). Additionally, there were chronic toxicity data for one freshwater chlorophyte, Scenedesmus subspicatus, which reported 4-day no observed effect level (NOEL) and EC50 (biomass yield, growth rate, area under the growth curve) values of 5000 μg L−1 and 106,000 μg L−1 , respectively54.
Ecological risk of haloxyfop
Haloxyfop has only recently been included in monitoring programs in GBR waters and detection frequencies (< 33%) and concentrations measured by passive sampling are generally low (< 1 ng L−1) in marine waters24. There are no current WQGVs for haloxyfop in freshwater or marine environments27, while the PGVs are based on toxicity data of a combination of one freshwater phototroph and five marine and freshwater heterotrophs36. However, the modelled distribution of these data indicated a poor data fit and subsequently resulted in low-reliability PGVs36. The PC99 PGV of 590 µg L−1 is an order of magnitude lower than the NEC value we report for C. muelleri of > 4570 µg L−1, indicating C. muelleri is well protected by this PGV and that environmental concentrations currently recorded do not pose a risk to this species in comparison to PSII herbicides. It should further be noted that haloxyfop may be less bioavailable in seawater due to its molecular structure. Haloxyfop contains a carboxyl group (COOH) which can result in complexation with Mg2+ and Ca2+ ions in seawater80, or stabilize the herbicide at the seawater:air interface81. These chemical properties could reduce the exposure and bioavailability of haloxyfop to marine species accounting for the low toxicities reported for the marine microalgae Rhodomonas salina26 and C. muelleri. Nevertheless, the acute and chronic toxicity data presented here will contribute towards deriving more reliable marine WQGVs for haloxyfop in the future, enabling the contribution of haloxyfop to the total herbicide risk to be assessed using ms-PAF.
Relationship between inhibition of effective quantum yield and growth
SSDs are currently developed using toxicity data from chronic exposure experiments, and ecologically relevant endpoints, such as inhibition of growth are preferred28. However, several studies have recommended the use of PAM fluorometry for estimating adverse biological effects of PSII herbicides26,40,82,83,84. In this study, the NEC and ECx values derived for SGR inhibition were all consistently higher than the respective NEC and ECx values estimated for inhibition in ΔF/Fm′ (Table 2). In fact, the direct comparison between EC50 values of each PSII herbicide calculated for SGR and ΔF/Fm′ inhibition revealed that SGR was on average 3-times less sensitive to PSII herbicide exposures than ΔF/Fm′ (Table 2). The relationship between herbicide inhibition of SGR and ΔF/Fm′ for marine microalgae has only been investigated in two earlier studies. Thomas et al.26 similarly reported that the SGR of the cryptophyte R. salina was on average 4-times less sensitive to PSII herbicide exposure than the photoinhibition endpoint. In a study by Magnusson et al.40 the relationship between SGR and ΔF/Fm′ inhibition by PSII herbicides was closer to 1:1 for two tropical benthic microalgae; Navicula sp. and Nephroselmis pyriformis. However, it is not necessarily expected that the reduced electron transport, due to the binding of PSII herbicides to the D1 protein is directly linked (1:1) to reduced growth rates for all taxa and experimental conditions. ΔF/Fm′ values are affected by actinic (ambient) light intensity and acclimation period of the test species and this in turn can affect the sensitvity of ΔF/Fm′ inhibition as an ecotoxicological endpoint85. Furthermore, the complex relationship between light-driven productivity and nutrient availability as well as species-specific physiologies make direct comparisons with prior studies more difficult. Nevertheless, the consistency of the linear relationship between toxicity thresholds based on ΔF/Fm′ and SGR for C. muelleri and three other marine species26,40 clearly demonstrated that inhibition of ΔF/Fm′ in PSII is directly linked to reduced growth in marine microalgae. This highlights the applicability of fluorescence microplate toxicity assays to quantify sub-lethal effects of PSII herbicides on microalgae. Indeed, the strength and consistency of this relationship, as well as the clear mechanistic link between inhibition of ΔF/Fm′ and growth rates indicates that for microalgae, inhibition of ΔF/Fm′ should be considered a valid bioindicator of ecological relevance and moreover, that chronic ΔF/Fm′ toxicity endpoints could contribute to deriving WQGVs for PSII herbicides in the future.
Conclusion
Although a revision of the current WQGVs has recently been proposed, most of the PGVs were derived from freshwater toxicity thresholds and consequently are of low reliability, signifying data gaps for tropical marine species, especially for marine phototrophs. Here, we demonstrated that exposures of the diatom C. muelleri towards PSII herbicides resulted in substantial reductions of ΔF/Fm′ within 24 h, which subsequently inhibited growth rates over 3-day chronic exposures. Inhibition in ΔF/Fm′ was on average 3-times more sensitive than inhibition in growth to PSII herbicide exposure, but was linearly related, highlighting the applicability of fluorescence microplate toxicity assays to quantify sub-lethal impacts of PSII herbicides on microalgae. These results are consistent with the responses of three other microalgal species26,40, supporting the notion that inhibition of ΔF/Fm′ could be considered a valid bioindicator of ecological relevance and moreover, that chronic ΔF/Fm′ toxicity endpoints could contribute to deriving future WQGVs for PSII herbicides. In contrast, the non-PSII herbicide haloxyfop did not affect SGR and ΔF/Fm′ in C. muelleri at very high concentrations, suggesting haloxyfop pose little risk to this microalga in the marine environment. While the toxicity thresholds (NECs and EC10s) derived here were all higher than concentrations detected in GBR monitoring programs, high-reliability WQGVs that underpin their regulation are generally lacking, especially for alternative herbicides. The toxicity thresholds (i.e. NECs) identified here for C. muelleri are therefore valuable contributions to the future derivation of high-reliability marine WQGVs for the PSII herbicide diuron, propazine, and tebuthiuron as well as the non-PSII herbicide haloxyfop, supporting improvements in cumulative risk assessments of herbicide mixtures using ms-PAF.
Methods
Diatom cultivation
The diatom Chaetoceros muelleri86 (strain CS-176) was purchased from the Australian National Algae Supply Service, Hobart. The genus Chaetoceros is considered as one of the most diverse genera of diatoms in the marine phytoplankton with a global distribution ranging from temperate to tropical regions87. Besides its importance as primary producer, this brackish-marine diatom is commonly used in aquaculture hatcheries for its high lipid content88. Prior to experimentation, cultures of C. muelleri were acclimatized under experimental conditions (below) for a period of two weeks and maintained in 500 mL Erlenmeyer flasks as batch cultures in exponential growth phase with weekly transfers of 70 mL algae suspension into 350 mL sterile culture medium. The culture medium was prepared from sterile 0.5 µm filtered seawater (FSW; pH 8.0, salinity 35.0 psu) enriched with Guillard’s f/2 marine medium89 (0.5 mL of AlgaBoost F/2, AusAqua in 1 L 0.5 µm-FSW). Cultures were continuously aerated and kept at 27.0 ± 1 °C and 35 psu. Cultures were exposed to a 12:12 h light:dark cycle with light supplied from two fluorescent tubes (Osram Lumilux Cool White 36 W) and irradiance adjusted to 100–110 μmol photons m–2 s–1.
Preparation of test solutions
Herbicides to be tested in this study were selected based on their application and detection rate in GBR monitoring programs and those currently lacking marine water quality guideline values. Diuron was chosen as a reference toxicant as its toxicity to a wide variety of microalgae is well studied26,40. Toxicant stock solutions were prepared using PESTANAL analytical grade products (Sigma-Aldrich, HPLC ≥ 98% purity): diuron (CAS 330-54-1), propazine (CAS 139-40-2), tebuthiuron (CAS 34014-18-1), haloxyfop (CAS 72619-32-0). Stock solutions of diuron (10 mg L−1), propazine (8.5 mg L−1), tebuthiuron (50 mg L−1), and haloxyfop (40 mg L−1) were prepared in sterile 500 mL Schott glass bottles using Milli-Q water or FSW and sonicated for a minimum of 2 h. A solvent carrier was used for the preparation of the diuron stock (HPLC-grade ethanol (< 0.001% (v/v) in exposure). No solvent carrier was used for tebuthiuron, propazine and haloxyfop.
Toxicity testing procedure
Chronic toxic effects of herbicides on the specific growth rate (SGR) of C. muelleri were tested in 72 h static exposure experiments according to the test procedure by Thomas et al.26 and based on OECD Test No. 20146. Initially, 15 mL of algae inoculum was taken from 4-day-old C. muelleri culture (approximately 2 × 106 cells mL−1) in exponential growth phase and washed with 15 mL sterile FSW by centrifugation in 50 mL falcon tubes at 1500×g for 5 min (Eppendorf Centrifuge 5810 R, Bio-strategy). The supernatant was decanted, and the remaining algae pellet homogenized in 30 mL FSW by vortexing. The centrifugation process was repeated three times prior to the start of each toxicity test. After the final washing, the cell pellet was re-suspended in 15 mL of sterile 0.5 µm-FSW and the cell density of the concentrated algae suspension was measured from two 500 µL sub-samples by flow cytometry. The desired inoculum was calculated to have a starting cell density of 3 × 103 cells mL−1 in the toxicity tests. Individual C. muelleri working suspensions for each herbicide treatment were prepared in 100 mL Schott glass bottles by adding the required algae inoculum and sterile 0.5 µm-FSW. Each Schott glass bottle was finally dosed with a range of herbicide concentrations (Table S-2). Five replicated aliquots of 10 mL were transferred from the individual 100 mL Schott glass bottles into sterile 20 mL glass scintillation vials and incubated at 27.5 ± 0.4 °C under a 12:12 h light:dark cycle at 90–100 μmol photons m–2 s–1 (Osram Lumilux Cool White 36 W). Vials were randomized and swirled daily. Bioassays for each herbicide were performed on different days with fresh algae, FSW and herbicide stocks. In each bioassay, a control (no herbicide) and reference (diuron, 4 µg L−1) treatment were included to indicate test consistency.
Cell density measurements
Sub-samples of 500 µL were taken from each replicate to measure cell densities of algal populations at 0 h and 72 h using a flow cytometer (BD Accuri C6, BD Biosciences, CA, USA) equipped with red and blue lasers (14.7 mW 640 nm Diode Red Laser 20 mW 488 nm Solid State Blue Laser) and standard filter setup26. The flow rate was set to 35 µL min−1, 16-µm core size with a sample volume of 50 µL. Cell densities were obtained by plotting a two-dimensional cytogram. A fixed gating was used around the viable (chlorophyll fluorescing) cells, which allowed for differentiation of non-algal particles (debris) and dead cells from viable cells, which typically represented 80–95% of particles counted (control treatment at 72 h). Aliquots were run in duplicates and an average taken of the number of events that occurred within the gated region. This process was then repeated for each replicate per treatment. Specific growth rates (SGR) were expressed as the logarithmic increase in cell density from day i (ti) to day j (tj) as per Eq. (1), where SGRi-j is the specific growth rate from time i to j; Xj is the cell density at day j and Xi is the cell density at day i46:
SGR relative to the control treatment was used to derive chronic effect values (EC10 and EC50) and no effect concentrations (NEC) for growth inhibition. A test was considered valid if the mean SGR of control replicates was ≥ 0.92 day−1, the percent coefficient of variation (% CV) of the average specific growth rate of control cultures did not exceed 10% and the pH of the control medium did not increase by more than 1-unit during the test46.
Chlorophyll fluorescence measurements
The effects of herbicide on chlorophyll fluorescence were measured as effective quantum yield (ΔF/Fm′) using imaging PAM fluorometry (I-PAM, Walz, Germany)83,90 following a single 12:12 h light:dark cycle (90–100 μmol photons m–2 s–1)26. Light-adapted minimum fluorescence (F) and maximum fluorescence measurements (Fm′) were taken in 48-well plates (Nunclon Delta, Thermo Scientific) from which the effective quantum yield was calculated as per Eq. (2) 90. An initial cell density of approximately 1 × 106 cells mL−1 was used to obtain ΔF/Fm′ measurements > 0.45 with the following I-PAM settings: actinic light = 1 (corresponding to photosynthetically active radiation (PAR) of 100–110 μmol photons m-2 s-1), measuring intensity = 9, gain = 1; damp = 2.
Prior to herbicide exposure a screening process of control treatments was performed to ensure consistent ΔF/Fm′ measurements > 0.45. Diuron was used as a referent toxicant (4 µg L−1) to monitor inhibition response between replicated algae cultures.
Chemical analyses
Physical and chemical characteristics of each treatment were measured at 0 h and 72 h including pH and salinity (LAQUAact-PC110 Meter, HORIBA Scientific) and dissolved oxygen (HQ30D Portable Meter, HACH). Temperature was logged in 10-min intervals over the total test duration (HOBO, Onset). Samples for chemical analysis were taken at start and end of herbicide exposure. Aliquots (1 mL) were transferred into 1.5 mL Liquid Chromatography amber glass vials and spiked with surrogate standards (i.e. diuron-D6, propazine-D6, and haloxyfop-D4) at a final concentration of 10 ng mL−1. Prior to analysis samples were stored at − 20 °C, defrosted and centrifuged. Herbicide concentrations were determined by HPLC–MS/MS using an SCIEX Triple Quad 6500 QTRAP mass spectrometer (SCIEX, Concord, Ontario, Canada) equipped with a TurboIonSpray probe22,23. The mass spectrometer was coupled to a Shimadzu Nexera X2 uHPLC system (Shimadzu Corp., Kyoto, Japan) using a Phenomenex Kinetex Biphenyl column (2.6 μm 50 × 2.1 mm 100 Å) for analyte separation. 5μL of sample was injected on to the column followed by a linear gradient starting at 10% B for 0.5 min, ramped to 100% B in 4.7 min then held at 100% for 4.0 min followed by equilibration at 10% B for 3.0 min (A = 1% methanol in Milli-Q water, B = 95% methanol in Milli-Q water, both containing 0.1% acetic acid). The mass spectrometer was operated in both positive and negative ion mode using a scheduled multiple reaction-monitoring method (sMRM). Positive samples were confirmed by retention time and by comparing transition intensity ratios between the sample and an appropriate calibration standard from the same run. The measured concentrations used for concentration–response modelling were derived from the geometric mean of measured start and end concentrations (time weighted average)26.
Data analysis
Statistical analyses and threshold estimates were based on measured herbicide concentrations (Table S-1). The inhibition of SGR and ΔF/Fm′ in C. muelleri by herbicides was quantified as per Eq. (3)46, where Xcontrol is the average SGR or ΔF/Fm′ of control and Xtreatment is the average SGR or ΔF/Fm′ of single treatments.
Concentrations that effectively inhibited SGR and ΔF/Fm′ by 10% or 50% (EC10 and EC50) and their 95% confidence intervals relative to the control treatment were calculated from nonlinear regression (Sigmoidal, 4-parameter) using GraphPad Prism V 8.0.
The relative potencies of each herbicides was determined using the relative equivalent potencies (ReP) compared to the reference herbicide diuron (EC50 diuron/EC50 herbicide)40. ReP values > 1 indicate potencies proportionally greater than diuron and ReP values < 1 indicate potencies less than diuron. SGR and ΔF/Fm′ data from haloxyfop experiments were analyzed using one-way analysis of variance (ANOVA) to determine any significant differences between treatments for each endpoint.
The estimations of no effect concentrations (NEC) that have no adverse effect on a species were calculated in R (Version 3.6.1) as per Thomas et al.26. Proportional decline in SGR (1-inhibition) was modelled as a function of log concentration of each herbicide using a Bayesian non-linear gaussian model using the R package jagsNEC91. This model has been specifically developed to derive no effect concentrations (NECs) and is defined by Eq. (4) 92:
\(\text{E[}{\mathrm{Y}}_{\mathrm{i}}\)|xi] is the mathematical expectation of Yi (the response, e.g. in this case the proportional decline in SGR) conditional on a given concentration xi. The model parameters for the generalised case are \(\alpha \) (the response at zero or low concentrations, also called ‘top’), \(-\beta \) (the rate of decay in the response after the NEC) and γ (the NEC value)92. For a gaussian Y, as used here, the model has the additional parameters Δ (an offset or intercept) and σ (the random error variance in Y) (see Thomas et al.26 for further details).
References
Carbery, K., Owen, R., Frickers, T., Otero, E. & Readman, J. Contamination of Caribbean coastal waters by the antifouling herbicide Irgarol 1051. Mar. Pollut. Bull. 52, 635–644. https://doi.org/10.1016/j.marpolbul.2005.10.013 (2006).
Hernández-Romero, A. H., Tovilla-Hernández, C., Malo, E. A. & Bello-Mendoza, R. Water quality and presence of pesticides in a tropical coastal wetland in southern Mexico. Mar. Pollut. Bull. 48, 1130–1141. https://doi.org/10.1016/j.marpolbul.2004.01.003 (2004).
Castillo, L. E., de la Cruz, E. & Ruepert, C. Ecotoxicology and pesticides in tropical aquatic ecosystems of Central America. Environ. Toxicol. Chem. 16, 41–51. https://doi.org/10.1002/etc.5620160104 (1997).
Basheer, C., Obbard, J. P. & Lee, H. K. Persistent organic pollutants in Singapore’s coastal marine environment: part I, seawater. Water Air Soil Pollut. 149, 295–313. https://doi.org/10.1023/A:1025689600993 (2003).
Ali, H. R. et al. Contamination of diuron in coastal waters around Malaysian Peninsular. Mar. Pollut. Bull. 85, 287–291. https://doi.org/10.1016/j.marpolbul.2014.05.049 (2014).
Okamura, H., Aoyama, I., Ono, Y. & Nishida, T. Antifouling herbicides in the coastal waters of western Japan. Mar. Pollut. Bull. 47, 59–67. https://doi.org/10.1016/S0025-326X(02)00418-6 (2003).
Roche, H., Salvat, B. & Ramade, F. Assessment of the pesticides pollution of coral reefs communities from French Polynesia. Rev. Ecol. https://hdl.handle.net/2042/55860 (2011).
Sarkar, S. K. et al. Occurrence, distribution and possible sources of organochlorine pesticide residues in tropical coastal environment of India: an overview. Environ. Int. 34, 1062–1071. https://doi.org/10.1016/j.envint.2008.02.010 (2008).
Devlin, M. M. et al. Advancing our understanding of the source, management, transport and impacts of pesticides on the Great Barrier Reef 2011–2015. Report for the Queensland Department of Environment and Heritage Protection. Tropical Water & Aquatic Ecosystem Research (TropWATER) Publication, James Cook University, Cairns, Australia (2015).
GBR. Great Barrier Reef Marine Park Authority 2019, Great Barrier Reef Outlook Report 2019, GBRMPA, Townsville. https://www.gbrmpa.gov.au/our-work/outlook-report-2019 (2019).
Brodie, J. et al. Terrestrial pollutant runoff to the Great Barrier Reef: an update of issues, priorities and management responses. Mar. Pollut. Bull. 65, 81–100. https://doi.org/10.1016/j.marpolbul.2011.12.012 (2012).
Lewis, S. E. et al. Herbicides: a new threat to the Great Barrier Reef. Environ. Pollut. 157, 2470–2484. https://doi.org/10.1016/j.envpol.2009.03.006 (2009).
RWQIP. Reef 2050 Water Quality Improvement Plan 2017–2022. Australian and Queensland Government. https://www.reefplan.qld.gov.au/__data/assets/pdf_file/0017/46115/reef-2050-water-quality-improvement-plan-2017-22.pdf (2018).
Shaw, M. et al. Monitoring pesticides in the Great Barrier Reef. Mar. Pollut. Bull. 60, 113–122. https://doi.org/10.1016/j.marpolbul.2009.08.026 (2010).
Grant, S. et al. Marine Monitoring Program: Annual report for inshore pesticide monitoring 2015–2016 (Report for the Great Barrier Reef Marine Park Authority, Great Barrier Reef Marine Park Authority, Townsville, Australia, 2017).
O’Brien, D. et al. Spatial and temporal variability in pesticide exposure downstream of a heavily irrigated cropping area: application of different monitoring techniques. J. Agric. Food Chem. 64, 3975–3989. https://doi.org/10.1021/acs.jafc.5b04710 (2016).
Radcliffe, J. Pesticide use in Australia. A review undertaken by the Australian Academy of Technological Sciences, Victoria, Australia. https://www.atse.org.au/ (2002).
Oettmeier, W. Herbicides of photosystems II. in Structure, Function and Molecular Biology (Barber, J., ed) (Elsevier, Amsterdam, 349–408). https://doi.org/10.1016/B978-0-444-89440-3.50018-7 (1992).
Lewis, S. E. et al. Using monitoring data to model herbicides exported to the Great Barrier Reef, Australia. in The 19th International Congress on Modelling and Simulation, Modelling and Simulation Society of Australia and New Zealand. MODSIM2011, 2051–2056 (2011).
Kennedy, K. et al. The influence of a season of extreme wet weather events on exposure of the World Heritage Area Great Barrier Reef to pesticides. Mar. Pollut. Bull. 64, 1495–1507. https://doi.org/10.1016/j.marpolbul.2012.05.014 (2012).
Kennedy, K. et al. Long term monitoring of photosystem II herbicides: correlation with remotely sensed freshwater extent to monitor changes in the quality of water entering the Great Barrier Reef, Australia. Mar. Pollut. Bull. 65, 292–305. https://doi.org/10.1016/j.marpolbul.2011.10.029 (2012).
Mercurio, P., Mueller, J. F., Eaglesham, G., Flores, F. & Negri, A. P. Herbicide persistence in seawater simulation experiments. PLoS ONE 10, e0136391. https://doi.org/10.1371/journal.pone.0136391 (2015).
Mercurio, P. et al. Degradation of herbicides in the tropical marine environment: influence of light and sediment. PLoS ONE 11, e0165890. https://doi.org/10.1371/journal.pone.0165890 (2016).
Gallen, C. et al. Marine Monitoring Program: Annual report for inshore pesticide monitoring 2017–18. Report for the Great Barrier Reef Marine Park Authority, Great Barrier Reef Marine Park Authority, Townsville, Australia. https://elibrary.gbrmpa.gov.au/jspui/handle/11017/3489 (2019).
Davis, A., Lewis, S., Brodie, J. & Benson, A. The potential benefits of herbicide regulation: a cautionary note for the Great Barrier Reef catchment area. Sci. Total Environ. 490, 81–92. https://doi.org/10.1016/j.scitotenv.2014.04.005 (2014).
Thomas, M. C., Flores, F., Kaserzon, S., Fisher, R. & Negri, A. P. Toxicity of ten herbicides to the tropical marine microalgae Rhodomonas salina. Sci. Rep. 10, 7612. https://doi.org/10.1038/s41598-020-64116-y (2020).
ANZG. Revised Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, Canberra, Australia. https://www.waterquality.gov.au/anz-guidelines/guideline-values/default/water-quality-toxicants/toxicants (2018).
Warne, M. St. J. et al. Revised method for deriving Australian and New Zealand water quality guideline values for toxicants: update of 2015 version. Prepared for the revision of the Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Governments and Australian state and territory governments, Canberra, Australia. 48 pp, https://www.waterquality.gov.au/anz-guidelines/guideline-values/derive/warne-method-derive. https://doi.org/10.13140/RG.2.2.36577.35686 (2018).
Warne, M. St. J., Smith, R. & Turner, R. Analysis of pesticide mixtures discharged to the lagoon of the Great Barrier Reef, Australia. Environ. Pollut. 265, 114088. https://doi.org/10.1016/j.envpol.2020.114088 (2020).
Magnusson, M., Heimann, K., Quayle, P. & Negri, A. P. Additive toxicity of herbicide mixtures and comparative sensitivity of tropical benthic microalgae. Mar. Pollut. Bull. 60, 1978–1987. https://doi.org/10.1016/j.marpolbul.2010.07.031 (2010).
Faust, M. et al. Predicting the joint algal toxicity of multi-component s-triazine mixtures at low-effect concentrations of individual toxicants. Aquat. Toxicol. 56, 13–32. https://doi.org/10.1016/S0166-445X(01)00187-4 (2001).
Wilkinson, A. D., Collier, C. J., Flores, F. & Negri, A. P. Acute and additive toxicity of ten photosystem-II herbicides to seagrass. Sci. Rep. 5, 17443. https://doi.org/10.1038/srep17443 (2015).
Traas, T. P. et al. The potentially affected fraction as a measure of ecological risk. in Species sensitivity distributions in ecotoxicology (L. Posthuma, & G. W. Suter, Eds.) (pp. 315–344). https://doi.org/10.1201/9781420032314-20 (2002).
Negri, A. P. et al. Adjusting tropical marine water quality guideline values for elevated ocean temperatures. Environ. Sci. Technol. 54, 1102–1110. https://doi.org/10.1021/acs.est.9b05961 (2019).
King, O., Smith, R., Mann, R. & Warne, M. St. J. Proposed aquatic ecosystem protection guideline values for pesticides commonly used in the Great Barrier Reef catchment area: Part 1 (amended): 2,4-D, Ametryn, Diuron, Glyphosate, Hexazinone, Imazapic, Imidacloprid, Isoxaflutole, Metolachlor, Metribuzin, Metsulfuron-methyl, Simazine, Tebuthiuron. Department of Environment and Science, Brisbane, Australia. https://www.publications.qld.gov.au/dataset/proposed-guideline-values-27-pesticides-used-in-the-gbr-catchment (2017).
King, O., Smith, R., Warne, M. St. J. & Mann, R. Proposed aquatic ecosystem protection guideline values for pesticides commonly used in the Great Barrier Reef catchment area: Part 2: Bromacil, Chlorothalonil, Fipronil, Fluometuron, Fluroxypyr, Haloxyfop, MCPA, Pendimethalin, Prometryn, Propazine, Propiconazole, Terbutryn, Triclopyr and Terbuthylazine. Department of Science, Information Technology and Innovation, Brisbane, Australia. https://www.publications.qld.gov.au/dataset/proposed-guideline-values-27-pesticides-used-in-the-gbr-catchment (2017).
Fleeger, J. W., Carman, K. R. & Nisbet, R. M. Indirect effects of contaminants in aquatic ecosystems. Sci. Total Environ. 317, 207–233. https://doi.org/10.1016/S0048-9697(03)00141-4 (2003).
Ralph, P. J. & Gademann, R. Rapid light curves: a powerful tool to assess photosynthetic activity. Aquat. Bot. 82, 222–237. https://doi.org/10.1016/j.aquabot.2005.02.006 (2005).
Schreiber, U. Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. in Chlorophyll a fluorescence (Springer, Dordrecht, 2004) 279–319.
Magnusson, M., Heimann, K. & Negri, A. P. Comparative effects of herbicides on photosynthesis and growth of tropical estuarine microalgae. Mar. Pollut. Bull. 56, 1545–1552. https://doi.org/10.1016/j.marpolbul.2008.05.023 (2008).
Sjollema, S. B. et al. Hazard and risk of herbicides for marine microalgae. Environ. Pollut. 187, 106–111. https://doi.org/10.1016/j.envpol.2013.12.019 (2014).
Muller, R. et al. Rapid exposure assessment of PSII herbicides in surface water using a novel chlorophyll a fluorescence imaging assay. Sci. Total Environ. 401, 51–59. https://doi.org/10.1016/j.scitotenv.2008.02.062 (2008).
Bengston-Nash, S. M., Quayle, P. A., Schreiber, U. & Muller, J. F. The selection of a model microalgal species as biomaterial for a novel aquatic phytotoxicity assay. Aquat. Toxicol. 72, 315–326. https://doi.org/10.1016/j.aquatox.2005.02.004 (2005).
Duggleby, R. G., McCourt, J. A. & Guddat, L. W. Structure and mechanism of inhibition of plant acetohydroxyacid synthase. Plant Physiol. Biochem. 46, 309–324. https://doi.org/10.1016/j.plaphy.2007.12.004 (2008).
Grossmann, K. Auxin herbicides: current status of mechanism and mode of action. Pest Manage. Sci. 66, 113–120. https://doi.org/10.1002/ps.1860 (2010).
OECD. Organisation for Economic Cooperation and Development (OECD) guidelines for the testing of chemicals: freshwater alga and cyanobacteria, growth inhibition test. Test No. 201, https://search.oecd.org/env/test-no-201-alga-growth-inhibition-test-9789264069923-en.htm (2011).
Lewis, K. A., Tzilivakis, J., Warner, D. J. & Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. Int. J. 22, 1050–1064. https://doi.org/10.1080/10807039.2015.1133242 (2016).
Rutherford, A. W. & Krieger-Liszkay, A. Herbicide-induced oxidative stress in photosystem II. Trends Biochem. Sci. 26, 648–653. https://doi.org/10.1016/S0968-0004(01)01953-3 (2001).
Chen, S., Yin, C., Strasser, R. J., Yang, C. & Qiang, S. Reactive oxygen species from chloroplasts contribute to 3-acetyl-5-isopropyltetramic acid-induced leaf necrosis of Arabidopsis thaliana. Plant Physiol. Biochem. 52, 38–51. https://doi.org/10.1016/j.plaphy.2011.11.004 (2012).
Chesworth, J., Donkin, M. & Brown, M. The interactive effects of the antifouling herbicides Irgarol 1051 and Diuron on the seagrass Zostera marina (L.). Aquat. Toxicol. 66, 293–305. https://doi.org/10.1016/j.aquatox.2003.10.002 (2004).
Jones, R. J. & Kerswell, A. P. Phytotoxicity of photosystem II (PSII) herbicides to coral. Mar. Ecol. Prog. Ser. 261, 149–159. https://doi.org/10.3354/meps261149 (2003).
van Dam, J. W., Negri, A. P., Mueller, J. F. & Uthicke, S. Symbiont-specific responses in foraminifera to the herbicide diuron. Mar. Pollut. Bull. 65, 373–383. https://doi.org/10.1016/j.marpolbul.2011.08.008 (2012).
Negri, A. P., Flores, F., Röthig, T. & Uthicke, S. Herbicides increase the vulnerability of corals to rising sea surface temperature. Limnol. Oceanogr. 56, 471–485. https://doi.org/10.4319/lo.2011.56.2.0471 (2011).
USEPA. ECOTOX User Guide: ECOTOXicology Database System. Version 5.0. United States Environmental Protection Agency. https://cfpub.epa.gov/ecotox/ (2019).
Bao, V. W., Leung, K. M., Qiu, J.-W. & Lam, M. H. Acute toxicities of five commonly used antifouling booster biocides to selected subtropical and cosmopolitan marine species. Mar. Pollut. Bull. 62, 1147–1151. https://doi.org/10.1016/j.marpolbul.2011.02.041 (2011).
Gatidou, G., Thomaidis, N. S. & Zhou, J. L. Fate of Irgarol 1051, diuron and their main metabolites in two UK marine systems after restrictions in antifouling paints. Environ. Int. 33, 70–77. https://doi.org/10.1016/j.envint.2006.07.002 (2007).
Jung, S. et al. Acute toxicity of organic antifouling biocides to phytoplankton Nitzschia pungens and zooplankton Artemia larvae. Mar. Pollut. Bull. 124, 811–818. https://doi.org/10.1016/j.marpolbul.2016.11.047 (2017).
Koutsaftis, A. & Aoyama, I. The interactive effects of binary mixtures of three antifouling biocides and three heavy metals against the marine algae Chaetoceros gracilis. Environ. Toxicol. Int. J. 21, 432–439. https://doi.org/10.1002/tox.20202 (2006).
Booij, P. et al. Identification of photosynthesis inhibitors of pelagic marine algae using 96-well plate microfractionation for enhanced throughput in effect-directed analysis. Environ. Sci. Technol. 48, 8003–8011. https://doi.org/10.1021/es405428t (2014).
DeLorenzo, M. E., Danese, L. E. & Baird, T. D. Influence of increasing temperature and salinity on herbicide toxicity in estuarine phytoplankton. Environ. Toxicol. 28, 359–371. https://doi.org/10.1002/tox.20726 (2013).
Devilla, R. A. et al. Impact of antifouling booster biocides on single microalgal species and on a natural marine phytoplankton community. Mar. Ecol. Prog. Ser. 286, 1–12. https://doi.org/10.3354/MEPS286001 (2005).
Mercurio, P. et al. Contribution of transformation products towards the total herbicide toxicity to tropical marine organisms. Sci. Rep. 8, 4808. https://doi.org/10.1038/s41598-018-23153-4 (2018).
Jones, R. The ecotoxicological effects of Photosystem II herbicides on corals. Mar. Pollut. Bull. 51, 495–506. https://doi.org/10.1016/j.marpolbul.2005.06.027 (2005).
Guasch, H. & Sabater, S. Light history influences the sensitivity to atrazine in periphytic algae. J. Phycol. 34, 233–241. https://doi.org/10.1046/j.1529-8817.1998.340233.x (1998).
Millie, D. F., Hersh, C. M. & Dionigi, C. P. Simazine-induced inhibition in photoacclimated populations of Anabaena circinalis (Cyanophyta). J. Phycol. 28, 19–26. https://doi.org/10.1111/j.0022-3646.1992.00019.x (1992).
Bérard, A. et al. Comparison of the ecotoxicological impact of the triazines Irgarol 1051 and atrazine on microalgal cultures and natural microalgal communities in Lake Geneva. Chemosphere 53, 935–944. https://doi.org/10.1016/S0045-6535(03)00674-X (2003).
Descolas-Gros, C. & Oriol, L. Variations in carboxylase activity in marine phytoplankton cultures. ß-carboxylation in carbon flux studies. Mar. Ecol. Prog. Ser. 85, 163–169 (1992).
Tang, J., Hoagland, K. D. & Siegfried, B. D. Uptake and bioconcentration of atrazine by selected freshwater algae. Environ. Toxicol. Chem. 17, 1085–1090. https://doi.org/10.1002/etc.5620170614 (1998).
Magnusson, M., Heimann, K., Ridd, M. & Negri, A. P. Chronic herbicide exposures affect the sensitivity and community structure of tropical benthic microalgae. Mar. Pollut. Bull. 65, 363–372. https://doi.org/10.1016/j.marpolbul.2011.09.029 (2012).
Tuchman, N. C., Schollett, M. A., Rier, S. T. & Geddes, P. Differential heterotrophic utilization of organic compounds by diatoms and bacteria under light and dark conditions. Hydrobiologia 561, 167–177. https://doi.org/10.1007/s10750-005-1612-4 (2006).
APVMA. Australian Pesticides and Veterinary Medicines Authority. https://apvma.gov.au/ (2019).
EPA. U.S. Environmental Protection Agency. https://www.epa.gov/pesticides (2020).
EC. European Commission. EU Pesticides database. https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/ (2020).
Novic, A. J. et al. Monitoring herbicide concentrations and loads during a flood event: a comparison of grab sampling with passive sampling. Environ. Sci. Technol. 51, 3880–3891. https://doi.org/10.1021/acs.est.6b02858 (2017).
Mercurio, P. Herbicide persistence and toxicity in the tropical marine environment. PhD University of Queensland. 148 p. https://doi.org/10.14264/uql.2016.722 (2016).
MacBean, C. The pesticide manual: a world compendium, 6th Edition 598–601 (British Crop Production Council (BCPC), Alton, 2012).
Huerlimann, R. & Heimann, K. Comprehensive guide to acetyl-carboxylases in algae. Crit. Rev. Biotechnol. 33, 49–65. https://doi.org/10.3109/07388551.2012.668671 (2013).
Kukorelli, G., Reisinger, P. & Pinke, G. ACCase inhibitor herbicides - selectivity, weed resistance and fitness cost: a review. Int. J. Pest Manage. 59, 165–173. https://doi.org/10.1080/09670874.2013.821212 (2013).
Huerlimann, R., Zenger, K. R., Jerry, D. R. & Heimann, K. Phylogenetic analysis of nucleus-encoded acetyl-CoA carboxylases targeted at the cytosol and plastid of algae. PLoS ONE https://doi.org/10.1371/journal.pone.0131099 (2015).
Tang, C. Y., Huang, Z. & Allen, H. C. Interfacial water structure and effects of Mg2+ and Ca2+ binding to the COOH headgroup of a palmitic acid monolayer studied by sum frequency spectroscopy. J. Phys. Chem. B 115, 34–40. https://doi.org/10.1021/jp1062447 (2010).
Brzozowska, A., Duits, M. H. & Mugele, F. Stability of stearic acid monolayers on Artificial Sea Water. Colloids Surf. Physicochem. Eng. Aspects 407, 38–48. https://doi.org/10.1016/j.colsurfa.2012.04.055 (2012).
Bengston-Nash, S. M., Schreiber, U., Ralph, P. J. & Muller, J. F. The combined SPE : ToxY-PAM phytotoxicity assay; application and appraisal of a novel biomonitoring tool for the aquatic environment. Biosens. Bioelectron. 20, 1443–1451. https://doi.org/10.1016/j.bios.2004.09.019 (2005).
Schreiber, U., Quayle, P., Schmidt, S., Escher, B. I. & Mueller, J. F. Methodology and evaluation of a highly sensitive algae toxicity test based on multiwell chlorophyll fluorescence imaging. Biosens. Bioelectron. 22, 2554–2563. https://doi.org/10.1016/j.bios.2006.10.018 (2007).
Haynes, D., Muller, J. & Carter, S. Pesticide and herbicide residues in sediments and seagrasses from the Great Barrier Reef World Heritage Area and Queensland coast. Mar. Pollut. Bull. 41, 279–287. https://doi.org/10.1016/s0025-326x(00)00097-7 (2000).
Ralph, P., Smith, R., Macinnis-Ng, C. & Seery, C. Use of fluorescence-based ecotoxicological bioassays in monitoring toxicants and pollution in aquatic systems. Toxicol. Environ. Chem. 89, 589–607. https://doi.org/10.1080/02772240701561593 (2007).
Lemmermann, E. D. grosse Waterneverstorfer Binnensee: Eine biologische Studie. Forsch. Biol. Station Plön 6, 166–205 (1896).
Li, Y. et al. Diversity in the globally distributed diatom genus Chaetoceros (Bacillariophyceae): three new species from warm-temperate waters. PLoS ONE https://doi.org/10.1371/journal.pone.0168887 (2017).
Helm, M. M., and Neil Bourne. Hatchery culture of bivalves: a practical manual. Ed. Alessandro Lovatelli. Fisheries Technical Paper 471. Food and Agriculture Organization of the United (FAO), 177 pp (2004).
Guillard, R. R. & Ryther, J. H. Studies of marine planktonic diatoms: I Cyclotellanana Hustedt, and Detonulaconfervacea (Cleve) Gran. Can. J. Microbiol. 8, 229–239. https://doi.org/10.1139/m62-029 (1962).
Schreiber, U., Müller, J. F., Haugg, A. & Gademann, R. New type of dual-channel PAM chlorophyll fluorometer for highly sensitive water toxicity biotests. Photosynth. Res. 74, 317–330. https://doi.org/10.1023/A:1021276003145 (2002).
Fisher, R., Ricardo, G., and Fox, D. jags NEC: A Bayesian No Effect Concentration (NEC) package. https://github.com/AIMS/NEC-estimation (2019).
Fox, D. R. A Bayesian approach for determining the no effect concentration and hazardous concentration in ecotoxicology. Ecotoxicol. Environ. Saf. 73, 123–131. https://doi.org/10.1016/j.ecoenv.2009.09.012 (2010).
Acknowledgements
The authors thank scientists from the Queensland Department of Environment and Science (DES) including Reinier Mann, Michael Warne and Rachael Smith who have provided valuable advice throughout this project. We also thank Carlos Alvarez-Roa from the Symbiont Culture Facility at the Australian Institute of Marine Science (AIMS) and Tom Barker from the National Sea Simulation (AIMS) for providing expertise for algae cultivation. Further, we would like to thank Rebecca Fisher for her advice regarding statistical analysis. This research was supported by the Australian Government’s National Environmental Science Program (NESP) Tropical Water Quality Hub Project 3.1.5 Ecotoxicology of pesticides on the Great Barrier Reef for guideline development and risk assessments. The authors acknowledge the Bindal and Wulgurukaba people as the Traditional Owners of the land where this work took place. We pay our respects to their elders past, present and emerging and we acknowledge their continuing spiritual connection to their land.
Author information
Authors and Affiliations
Contributions
M.C.T., F.F. and A.P.N. designed the experiment. M.C.T. and F.F. performed experiments. M.C.T. analyzed the data with input from F.F. and A.P.N. S.K. and T.A.R. performed chemical analyses of samples. M.C.T. wrote the manuscript with input from A.P.N., F.F., S.K., and T.A.R..
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thomas, M.C., Flores, F., Kaserzon, S. et al. Toxicity of the herbicides diuron, propazine, tebuthiuron, and haloxyfop to the diatom Chaetoceros muelleri. Sci Rep 10, 19592 (2020). https://doi.org/10.1038/s41598-020-76363-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76363-0
This article is cited by
-
Toxicity of herbicides to the marine microalgae Tisochrysis lutea and Tetraselmis sp.
Scientific Reports (2024)
-
Effects of triazine herbicide terbutryn on physiological responses and gene expression in Alexandrium catenella
Journal of Applied Phycology (2023)
-
Expression analyses of stress-responsive genes in the hermatypic coral Acropora tenuis and its symbiotic dinoflagellates after exposure to the herbicide Diuron
Marine Life Science & Technology (2023)
-
Effects of currently used marine antifouling paint biocides on green fluorescent proteins in Anemonia viridis
Journal of Fluorescence (2022)
-
Monoculture and co-culture tests of the toxicity of four typical herbicides on growth, photosynthesis and oxidative stress responses of the marine diatoms Pseudo-nitzschia mannii and Chaetoceros decipiens
Ecotoxicology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.