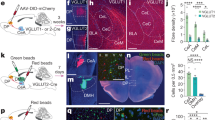
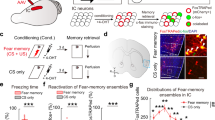
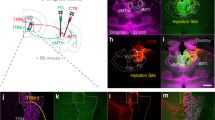
Abstract
Flight, an active fear response to imminent threat, is dependent on the rapid risk assessment of sensory information processed by the cortex. The thalamic reticular nucleus (TRN) filters information between the cortex and the thalamus, but whether it participates in the regulation of flight behavior remains largely unknown. Here, we report that activation of parvalbumin-expressing neurons in the limbic TRN, but not those in the sensory TRN, mediates flight. Glutamatergic inputs from the cingulate cortex (Cg) selectively activate the limbic TRN, which in turn inhibits the intermediodorsal thalamic nucleus (IMD). Activation of this Cg→limbic TRN→IMD circuit results in inhibition of the IMD and produces flight behavior. Conversely, removal of inhibition onto the IMD results in more freezing and less flight, suggesting that the IMD may function as a pro-freeze center. Overall, these findings reveal a novel corticothalamic circuit through the TRN that controls the flight response.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All relevant data supporting the present study are available from the lead corresponding author upon reasonable request.
Code availability
All relevant codes supporting the present study are available from the lead corresponding author upon reasonable request.
References
Anderson, D. J. & Adolphs, R. A framework for studying emotions across species. Cell 157, 187–200 (2014).
LeDoux, J. Rethinking the emotional brain. Neuron 73, 653–676 (2012).
Gross, C. T. & Canteras, N. S. The many paths to fear. Nat. Rev. Neurosci. 13, 651–658 (2012).
Keay, K. A. & Bandler, R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci. Biobehav. Rev. 25, 669–678 (2001).
Li, H. et al. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 16, 332–339 (2013).
Penzo, M. A. et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 (2015).
Isosaka, T. et al. Htr2a-expressing cells in the central amygdala control the hierarchy between innate and learned fear. Cell 163, 1153–1164 (2015).
Tovote, P. et al. Midbrain circuits for defensive behaviour. Nature 534, 206–212 (2016).
Fadok, J. P. et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature 542, 96–100 (2017).
Wang, L., Chen, I. Z. & Lin, D. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron 85, 1344–1358 (2015).
Clemence, A. E. & Mitrofanis, J. Cytoarchitectonic heterogeneities in the thalamic reticular nucleus of cats and ferrets. J. Comp. Neurol. 322, 167–180 (1992).
Crick, F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc. Natl Acad. Sci. USA 81, 4586–4590 (1984).
Guillery, R. W., Feig, S. L. & Lozsadi, D. A. Paying attention to the thalamic reticular nucleus. Trends Neurosci. 21, 28–32 (1998).
Halassa, M. M. & Acsady, L. Thalamic inhibition: diverse sources, diverse scales. Trends Neurosci. 39, 680–693 (2016).
Halassa, M. M. et al. State-dependent architecture of thalamic reticular subnetworks. Cell 158, 808–821 (2014).
Wimmer, R. D. et al. Thalamic control of sensory selection in divided attention. Nature 526, 705–709 (2015).
Ahrens, S. et al. ErbB4 regulation of a thalamic reticular nucleus circuit for sensory selection. Nat. Neurosci. 18, 104–111 (2015).
Zhu, Y., Jiang, X. & Ji, W. The mechanism of cortico-striato-thalamo-cortical neurocircuitry in response inhibition and emotional responding in attention deficit hyperactivity disorder with comorbid disruptive behavior disorder. Neurosci. Bull. 34, 566–572 (2018).
Marlinski, V., Sirota, M. G. & Beloozerova, I. N. Differential gating of thalamocortical signals by reticular nucleus of thalamus during locomotion. J. Neurosci. 32, 15823–15836 (2012).
Steriade, M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci. 28, 317–324 (2005).
Tovote, P., Fadok, J. P. & Luthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331 (2015).
Zikopoulos, B. & Barbas, H. Pathways for emotions and attention converge on the thalamic reticular nucleus in primates. J. Neurosci. 32, 5338–5350 (2012).
Pinault, D. The thalamic reticular nucleus: structure, function and concept. Brain Res. Brain Res. Rev. 46, 1–31 (2004).
Zikopoulos, B. & Barbas, H. Prefrontal projections to the thalamic reticular nucleus form a unique circuit for attentional mechanisms. J. Neurosci. 26, 7348–7361 (2006).
Schmitt, L. I. et al. Thalamic amplification of cortical connectivity sustains attentional control. Nature 545, 219–223 (2017).
Han, C. J. et al. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proc. Natl Acad. Sci. USA 100, 13087–13092 (2003).
Shenhav, A., Botvinick, M. M. & Cohen, J. D. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron 79, 217–240 (2013).
Nili, U., Goldberg, H., Weizman, A. & Dudai, Y. Fear thou not: activity of frontal and temporal circuits in moments of real-life courage. Neuron 66, 949–962 (2010).
Makinson, C. D. & Huguenard, J. R. Attentional flexibility in the thalamus: now we’re getting SOMwhere. Nat. Neurosci. 18, 2–4 (2015).
Clemente-Perez, A. et al. Distinct thalamic reticular cell types differentially modulate normal and pathological cortical rhythms. Cell Rep. 19, 2130–2142 (2017).
Yang, H. et al. Laterodorsal tegmentum interneuron subtypes oppositely regulate olfactory cue-induced innate fear. Nat. Neurosci. 19, 283–289 (2016).
Bliss, T. V., Collingridge, G. L., Kaang, B. K. & Zhuo, M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat. Rev. Neurosci. 17, 485–496 (2016).
Pinault, D. & Deschenes, M. Projection and innervation patterns of individual thalamic reticular axons in the thalamus of the adult rat: a three-dimensional, graphic, and morphometric analysis. J. Comp. Neurol. 391, 180–203 (1998).
Jones, E. G. Some aspects of the organization of the thalamic reticular complex. J. Comp. Neurol. 162, 285–308 (1975).
Lozsadi, D. A. Organization of cortical afferents to the rostral, limbic sector of the rat thalamic reticular nucleus. J. Comp. Neurol. 341, 520–533 (1994).
Beier, K. T. et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162, 622–634 (2015).
Schwarz, L. A. et al. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524, 88–92 (2015).
Matyas, F., Lee, J., Shin, H. S. & Acsady, L. The fear circuit of the mouse forebrain: connections between the mediodorsal thalamus, frontal cortices and basolateral amygdala. Eur. J. Neurosci. 39, 1810–1823 (2014).
Conley, M., Kupersmith, A. C. & Diamond, I. T. The organization of projections from subdivisions of the auditory cortex and thalamus to the auditory sector of the thalamic reticular nucleus in galago. Eur. J. Neurosci. 3, 1089–1103 (1991).
Crabtree, J. W. & Killackey, H. P. The topographic organization and axis of projection within the visual sector of the rabbit’s thalamic reticular nucleus. Eur. J. Neurosci. 1, 94–109 (1989).
Crabtree, J. W. The somatotopic organization within the cat’s thalamic reticular nucleus. Eur. J. Neurosci. 4, 1352–1361 (1992).
Montero, V. M., Guillery, R. W. & Woolsey, C. N. Retinotopic organization within the thalamic reticular nucleus demonstrated by a double label autoradiographic technique. Brain Res. 138, 407–421 (1977).
McCafferty, C. et al. Cortical drive and thalamic feed-forward inhibition control thalamic output synchrony during absence seizures. Nat. Neurosci. 21, 744–756 (2018).
Çavdar, S. et al. Comparison of numbers of interneurons in three thalamic nuclei of normal and epileptic rats. Neurosci. Bull. 30, 451–460 (2014).
Yu, K., Garcia da Silva, P., Albeanu, D. F. & Li, B. Central amygdala somatostatin neurons gate passive and active defensive behaviors. J. Neurosci. 36, 6488–6496 (2016).
Fan, Z. & Hu, H. Medial prefrontal cortex excitation/inhibition balance and schizophrenia-like behaviors regulated by thalamic inputs to interneurons. Biol. Psychiatry 83, 630–631 (2018).
Corcoran, K. A. & Quirk, G. J. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J. Neurosci. 27, 840–844 (2007).
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Boeke, E. A., Moscarello, J. M., LeDoux, J. E., Phelps, E. A. & Hartley, C. A. Active avoidance: neural mechanisms and attenuation of pavlovian conditioned responding. J. Neurosci. 37, 4808–4818 (2017).
Li, Y. et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat. Commun. 7, 10503 (2016).
Iyer, S. M. et al. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat. Biotechnol. 32, 274–278 (2014).
Yang, S. B. et al. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron 75, 425–436 (2012).
Li, K. X. et al. Neuregulin 1 regulates excitability of fast-spiking neurons through Kv1.1 and acts in epilepsy. Nat. Neurosci. 15, 267–273 (2012).
Lerner, T. N. et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015).
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates 3rd edn (Academic Press, 2007).
Acknowledgements
The authors thank J. Hu (Shanghai Technology University, China) for providing the AAV2/9-EF1a-DIO-mGFP virus, E. J. Kremer (IGMM, Montpellier, France) for providing the CAV virus, L. Luo (Stanford, CA, USA) for providing the Flp-dependent AAV helpers. This work was supported by the National Natural Science Foundation of China (grant nos. 31430034, 31871070, and 91432306), the National Key Research and Development Plan of the Ministry of Science and Technology of China (grant no. 2016YF0501000), the Non-Profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (grant nos. 2017PT31038 and 2018PT31041), Funds for Creative Research Groups of China from the National Natural Science Foundation of China (grant no. 81521062), the Program for Changjiang Scholars and Innovative Research Team in University, and the programme of introducing talents of discipline to universities (grant no. B13026) to X.-M.L.
Author information
Authors and Affiliations
Contributions
P.D. and X.-M.L. designed the project, and P.D. and H.W. performed virus or drug injections, optogenetic behavior, and electrophysiology experiments, and collected and analyzed the data. X.-T.Z. and J.-H.G. helped collect the data. P.D., P.J., X.-F.S., S.L., and Y.H. performed immunohistochemistry and quantitatively analyzed the imaging data. X.-B.H. generated RV and pseudo-RV, and F.-Q.X. supervised the retrograde virus labeling experiments. P.D., Y.L., J.C., S.D., H.W., and X.-M.L. interpreted the results and commented on the manuscript. P.D., H.W., H.L., J.C., and X.-M.L. wrote or edited the manuscript. X.-M.L. supervised all aspects of the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Neuroscience thanks Laszlo Acsady, Wulf Haubensak, and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–16
Supplementary Video 1
Fiber photometry recording of limbic TRN PV+ neurons during conditioned flight behavior. A representative trial of conditioned flight behavior in day 3 during fiber photometry recording.
Supplementary Video 2
Inhibition of limbic TRN PV+ neurons during conditioned flight behavior. Optogenetic inhibition of limbic TRN PV+ neurons decrease flight behavior.
Supplementary Video 3
Optogenetic activation of limbic TRN PV+ neurons during conditioned flight behavior. Optogenetic activation of limbic TRN PV+ neurons induce flight behavior.
Rights and permissions
About this article
Cite this article
Dong, P., Wang, H., Shen, XF. et al. A novel cortico-intrathalamic circuit for flight behavior. Nat Neurosci 22, 941–949 (2019). https://doi.org/10.1038/s41593-019-0391-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-019-0391-6
This article is cited by
-
A molecularly defined amygdala-independent tetra-synaptic forebrain-to-hindbrain pathway for odor-driven innate fear and anxiety
Nature Neuroscience (2024)
-
Top-down control of flight by a non-canonical cortico-amygdala pathway
Nature (2024)
-
The endocannabinoid N-arachidonoyl dopamine is critical for hyperalgesia induced by chronic sleep disruption
Nature Communications (2023)
-
Region-selective control of the thalamic reticular nucleus via cortical layer 5 pyramidal cells
Nature Neuroscience (2023)
-
Research Progress on the Mechanisms of Central Post-Stroke Pain: A Review
Cellular and Molecular Neurobiology (2023)