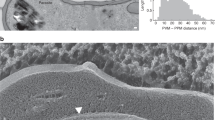
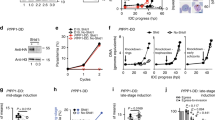
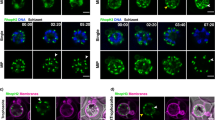
Abstract
Intraerythrocytic malaria parasites reside within a parasitophorous vacuolar membrane (PVM) generated during host cell invasion1. Erythrocyte remodelling and parasite metabolism require the export of effector proteins and transport of small molecules across this barrier between the parasite surface and host cell cytosol2,3. Protein export across the PVM is accomplished by the Plasmodium translocon of exported proteins (PTEX) consisting of three core proteins, the AAA+ ATPase HSP101 and two additional proteins known as PTEX150 and EXP24. Inactivation of HSP101 and PTEX150 arrests protein export across the PVM5,6, but the contribution of EXP2 to parasite biology is not well understood7. A nutrient permeable channel in the PVM has also been characterized electrophysiologically, but its molecular identity is unknown8,9. Here, using regulated gene expression, mutagenesis and cell-attached patch-clamp measurements, we show that EXP2, the putative membrane-spanning channel of PTEX4,10,11,12,13,14, serves dual roles as a protein-conducting channel in the context of PTEX and as a channel able to facilitate nutrient passage across the PVM independent of HSP101. Our data suggest a dual functionality for a channel operating in its endogenous context.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lingelbach, K. & Joiner, K. A. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J. Cell Sci. 111, 1467–1475 (1998).
Desai, S. A. Ion and nutrient uptake by malaria parasite-infected erythrocytes. Cell. Microbiol. 14, 1003–1009 (2012).
Sherling, E. S. & van Ooij, C. Host cell remodeling by pathogens: the exomembrane system in Plasmodium-infected erythrocytes. FEMS Microbiol. Rev. 40, 701–721 (2016).
de Koning-Ward, T. F. et al. A newly discovered protein export machine in malaria parasites. Nature 459, 945–949 (2009).
Beck, J. R., Muralidharan, V., Oksman, A. & Goldberg, D. E. PTEX component HSP101 mediates export of diverse malaria effectors into host erythrocytes. Nature 511, 592–595 (2014).
Elsworth, B. et al. PTEX is an essential nexus for protein export in malaria parasites. Nature 511, 587–591 (2014).
Kalanon, M. et al. The Plasmodium translocon of exported proteins component EXP2 is critical for establishing a patent malaria infection in mice. Cell. Microbiol. 18, 399–412 (2016).
Desai, S. A., Krogstad, D. J. & McCleskey, E. W. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature 362, 643–646 (1993).
Desai, S. A. & Rosenberg, R. L. Pore size of the malaria parasite’s nutrient channel. Proc. Natl Acad. Sci. USA 94, 2045–2049 (1997).
Johnson, D. et al. Characterization of membrane proteins exported from Plasmodium falciparum into the host erythrocyte. Parasitology 109, 1–9 (1994).
Bullen, H. E. et al. Biosynthesis, localization, and macromolecular arrangement of the Plasmodium falciparum translocon of exported proteins (PTEX). J. Biol. Chem. 287, 7871–7884 (2012).
Hakamada, K., Watanabe, H., Kawano, R., Noguchi, K. & Yohda, M. Expression and characterization of the Plasmodium translocon of the exported proteins component EXP2. Biochem. Biophys. Res. Comm. 482, 700–705 (2017).
Gold, D. A. et al. The Toxoplasma dense granule proteins GRA17 and GRA23 mediate the movement of small molecules between the host and the parasitophorous vacuole. Cell Host Microbe 17, 642–652 (2015).
Mesen-Ramirez, P. et al. Stable translocation intermediates jam global protein export in Plasmodium falciparum parasites and link the PTEX component EXP2 with translocation activity. PLoS Pathog. 12, e1005618 (2016).
Ganesan, S. M., Falla, A., Goldfless, S. J., Nasamu, A. S. & Niles, J. C. Synthetic RNA–protein modules integrated with native translation mechanisms to control gene expression in malaria parasites. Nat. Commun. 7, 10727 (2016).
Nasamu, A. S. et al. Plasmepsins IX and X are essential and druggable mediators of malaria parasite egress and invasion. Science 358, 518–522 (2017).
Glushakova, S. et al. Hemoglobinopathic erythrocytes affect the intraerythrocytic multiplication of Plasmodium falciparum in vitro. J. Infect. Dis. 210, 1100–1109 (2014).
Goldberg, D. E. & Cowman, A. F. Moving in and renovating: exporting proteins from Plasmodium into host erythrocytes. Nat. Rev. Microbiol. 8, 617–621 (2010).
Kulzer, S., Petersen, W., Baser, A., Mandel, K. & Przyborski, J. M. Use of self-assembling GFP to determine protein topology and compartmentalisation in the Plasmodium falciparum-infected erythrocyte. Mol. Biochem. Parasitol. 187, 87–90 (2013).
Otto, T. D. et al. New insights into the blood-stage transcriptome of Plasmodium falciparum using RNA-seq. Mol. Microbiol. 76, 12–24 (2010).
Boddey, J. A. & Cowman, A. F. Plasmodium nesting: remaking the erythrocyte from the inside out. Annu. Rev. Microbiol. 67, 243–269 (2013).
Schwab, J. C., Beckers, C. J. & Joiner, K. A. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc. Natl Acad. Sci. USA 91, 509–513 (1994).
Charpian, S. & Przyborski, J. M. Protein transport across the parasitophorous vacuole of Plasmodium falciparum: into the great wide open. Traffic 9, 157–165 (2008).
Glushakova, S. et al. Exploitation of a newly-identified entry pathway into the malaria parasite-infected erythrocyte to inhibit parasite egress. Sci. Rep. 7, 12250 (2017).
Ito, D., Schureck, M. A. & Desai, S. A. An essential dual-function complex mediates erythrocyte invasion and channel-mediated nutrient uptake in malaria parasites. eLife 6, e23485 (2017).
Garten, M. et al. Whole-GUV patch-clamping. Proc. Natl Acad. Sci. USA 114, 328–333 (2017).
Levadny, V., Aguilella, V. M. & Belaya, M. Access resistance of a single conducting membrane channel. Biochim. Biophys. Acta 1368, 338–342 (1998).
Hyun, C., Rollings, R. & Li, J. Probing access resistance of solid-state nanopores with a scanning probe microscope tip. Small 8, 385–392 (2012).
Ho, C.-M. et al. Malaria parasite translocon structure and mechanism of effector export. Nature https://doi.org/10.1038/s41586-018-0469-4 (2018).
Klemba, M., Beatty, W., Gluzman, I. & Goldberg, D. E. Trafficking of plasmepsin II to the food vacuole of the malaria parasite Plasmodium falciparum. J. Cell Biol. 164, 47–56 (2004).
Spillman, N. J., Beck, J. R., Ganesan, S. M., Niles, J. C. & Goldberg, D. E. The chaperonin TRiC forms an oligomeric complex in the malaria parasite cytosol. Cell. Microbiol. 19, e12719 (2017).
Ganesan, S. M. et al. Yeast dihydroorotate dehydrogenase as a new selectable marker for Plasmodium falciparum transfection. Mol. Biochem. Parasitol. 177, 29–34 (2011).
Adjalley, S. H. et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc. Natl Acad. Sci. USA 108, E1214–E1223 (2011).
Muralidharan, V., Oksman, A., Pal, P., Lindquist, S. & Goldberg, D. E. Plasmodium falciparum heat shock protein 110 stabilizes the asparagine repeat-rich parasite proteome during malarial fevers. Nat. Commun. 3, 1310 (2012).
Nkrumah, L. J. et al. Efficient site-specific integration in Plasmodium falciparum chromosomes mediated by mycobacteriophage Bxb1 integrase. Nat. Methods 3, 615–621 (2006).
Hall, R. et al. Antigens of the erythrocytes stages of the human malaria parasite Plasmodium falciparum detected by monoclonal antibodies. Mol. Biochem. Parasitol. 7, 247–265 (1983).
Rock, E. P. et al. Comparative analysis of the Plasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology 95, 209–227 (1987).
Blisnick, T. et al. Pfsbp1, a Maurer’s cleft Plasmodium falciparum protein, is associated with the erythrocyte skeleton. Mol. Biochem. Parasitol. 111, 107–121 (2000).
Guggisberg, A. M. et al. A sugar phosphatase regulates the methylerythritol phosphate (MEP) pathway in malaria parasites. Nat. Commun. 5, 4467 (2014).
Glushakova, S., Yin, D., Li, T. & Zimmerberg, J. Membrane transformation during malaria parasite release from human red blood cells. Curr. Biol. 15, 1645–1650 (2005).
Edelstein, A. D. et al. Advanced methods of microscope control using muManager software. J. Biol. Methods 1, e10 (2014).
Hodgkin, A. L. & Huxley, A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952).
Fisher, R. A. On the interpretation of χ2 from contingency tables, and the calculation of P. J. R. Stat. Soc. 85, 87–94 (1922).
Wilson, E. B. Probable inference, the law of succession, and statistical inference. J. Am. Stat. Assoc. 22, 209–212 (1927).
Acknowledgements
This work was supported by NIH grants HL133453 to J.R.B. and AI47798 to D.E.G. and by the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. We thank S. Glushakova for creating and applying the parasite liberation technique, for performing the IMF experiment and for many helpful conversations throughout the course of this project. We thank J. McBride, D. Cavanagh and EMRR for the EXP2 antibody, D. Taylor for HRP2 antibody, C. Braun-Breton for SBP1 antibody, W. Beatty for assistance with electron microscopy, P. Gurnev for the buffer conductivity measurement, P. S. Blank for helpful suggestions for the statistical analysis of the patch-clamp data and B. Vaupel for technical assistance.
Author information
Authors and Affiliations
Contributions
M.G., J.Z., D.E.G. and J.R.B. conceived and designed experiments. M.G. and J.R.B. performed the experiments. J.R.B. generated and analysed the parasite strains. M.G. performed patch-clamp analysis. A.S.N. generated the pEXP2apt plasmid. J.C.N. contributed reagents. M.G., J.Z., D.E.G. and J.R.B. analysed the data and wrote the manuscript. All authors discussed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–13, Supplementary References
Rights and permissions
About this article
Cite this article
Garten, M., Nasamu, A.S., Niles, J.C. et al. EXP2 is a nutrient-permeable channel in the vacuolar membrane of Plasmodium and is essential for protein export via PTEX. Nat Microbiol 3, 1090–1098 (2018). https://doi.org/10.1038/s41564-018-0222-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-018-0222-7
This article is cited by
-
Composition and stage dynamics of mitochondrial complexes in Plasmodium falciparum
Nature Communications (2021)
-
An integrated platform for genome engineering and gene expression perturbation in Plasmodium falciparum
Scientific Reports (2021)
-
Plasmodium translocon component EXP2 facilitates hepatocyte invasion
Nature Communications (2020)
-
The parasitophorous vacuole of the blood-stage malaria parasite
Nature Reviews Microbiology (2020)
-
Contacting domains segregate a lipid transporter from a solute transporter in the malarial host–parasite interface
Nature Communications (2020)