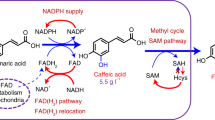
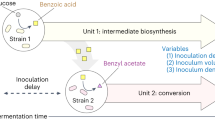
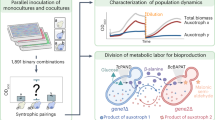
Abstract
Metabolic engineering of microorganisms such as Escherichia coli and Saccharomyces cerevisiae to produce high-value natural metabolites is often done through functional reconstitution of long metabolic pathways. Problems arise when parts of pathways require specialized environments or compartments for optimal function. Here we solve this problem through co-culture of engineered organisms, each of which contains the part of the pathway that it is best suited to hosting. In one example, we divided the synthetic pathway for the acetylated diol paclitaxel precursor into two modules, expressed in either S. cerevisiae or E. coli, neither of which can produce the paclitaxel precursor on their own. Stable co-culture in the same bioreactor was achieved by designing a mutualistic relationship between the two species in which a metabolic intermediate produced by E. coli was used and functionalized by yeast. This synthetic consortium produced 33 mg/L oxygenated taxanes, including a monoacetylated dioxygenated taxane. The same method was also used to produce tanshinone precursors and functionalized sesquiterpenes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ajikumar, P.K. et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science 330, 70–74 (2010).
Paddon, C.J. et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 496, 528–532 (2013).
Alonso-Gutierrez, J. et al. Metabolic engineering of Escherichia coli for limonene and perillyl alcohol production. Metab. Eng. 19, 33–41 (2013).
Ajikumar, P.K. et al. Terpenoids: opportunities for biosynthesis of natural product drugs using engineered microorganisms. Mol. Pharm. 5, 167–190 (2008).
Hefferon, K. Plant-derived pharmaceuticals for the developing world. Biotechnol. J. 8, 1193–1202 (2013).
Melnik, S. & Stoger, E. Green factories for biopharmaceuticals. Curr. Med. Chem. 20, 1038–1046 (2013).
Chang, M.C., Eachus, R.A., Trieu, W., Ro, D.K. & Keasling, J.D. Engineering Escherichia coli for production of functionalized terpenoids using plant P450s. Nat. Chem. Biol. 3, 274–277 (2007).
Agapakis, C.M., Boyle, P.M. & Silver, P.A. Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat. Chem. Biol. 8, 527–535 (2012).
Smid, E.J. & Lacroix, C. Microbe-microbe interactions in mixed culture food fermentations. Curr. Opin. Biotechnol. 24, 148–154 (2013).
Fredrickson, A.G. & Stephanopoulos, G. Microbial competition. Science 213, 972–979 (1981).
Davison, B.H. & Stephanopoulos, G. Effect of pH oscillations on a competing mixed culture. Biotechnol. Bioeng. 28, 1127–1137 (1986).
Bayer, T.S. et al. Synthesis of methyl halides from biomass using engineered microbes. J. Am. Chem. Soc. 131, 6508–6515 (2009).
Minty, J.J. et al. Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc. Natl. Acad. Sci. USA 110, 14592–14597 (2013).
Xia, T., Eiteman, M.A. & Altman, E. Simultaneous utilization of glucose, xylose and arabinose in the presence of acetate by a consortium of Escherichia coli strains. Microb. Cell Fact. 11, 77 (2012).
Guerra-Bubb, J., Croteau, R. & Williams, R.M. The early stages of taxol biosynthesis: an interim report on the synthesis and identification of early pathway metabolites. Nat. Prod. Rep. 29, 683–696 (2012).
Jennewein, S., Long, R.M., Williams, R.M. & Croteau, R. Cytochrome p450 taxadiene 5alpha-hydroxylase, a mechanistically unusual monooxygenase catalyzing the first oxygenation step of taxol biosynthesis. Chem. Biol. 11, 379–387 (2004).
Hefner, J. et al. Cytochrome P450-catalyzed hydroxylation of taxa-4(5),11(12)-diene to taxa-4(20),11(12)-dien-5alpha-ol: the first oxygenation step in taxol biosynthesis. Chem. Biol. 3, 479–489 (1996).
Nowak, M.A. Five rules for the evolution of cooperation. Science 314, 1560–1563 (2006).
Eiteman, M.A. & Altman, E. Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol. 24, 530–536 (2006).
Sun, J. et al. Cloning and characterization of a panel of constitutive promoters for applications in pathway engineering in Saccharomyces cerevisiae. Biotechnol. Bioeng. 109, 2082–2092 (2012).
Blazeck, J., Garg, R., Reed, B. & Alper, H.S. Controlling promoter strength and regulation in Saccharomyces cerevisiae using synthetic hybrid promoters. Biotechnol. Bioeng. 109, 2884–2895 (2012).
De Virgilio, C. et al. Cloning and disruption of a gene required for growth on acetate but not on ethanol: the acetyl-coenzyme A synthetase gene of Saccharomyces cerevisiae. Yeast 8, 1043–1051 (1992).
Kratzer, S. & Schuller, H.J. Transcriptional control of the yeast acetyl-CoA synthetase gene, ACS1, by the positive regulators CAT8 and ADR1 and the pleiotropic repressor UME6. Mol. Microbiol. 26, 631–641 (1997).
Causey, T.B., Zhou, S., Shanmugam, K.T. & Ingram, L.O. Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc. Natl. Acad. Sci. USA 100, 825–832 (2003).
Walker, K., Schoendorf, A. & Croteau, R. Molecular cloning of a taxa-4(20),11(12)-dien-5alpha-ol-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Arch. Biochem. Biophys. 374, 371–380 (2000).
Schoendorf, A., Rithner, C.D., Williams, R.M. & Croteau, R.B. Molecular cloning of a cytochrome P450 taxane 10 beta-hydroxylase cDNA from Taxus and functional expression in yeast. Proc. Natl. Acad. Sci. USA 98, 1501–1506 (2001).
Wheeler, A.L. et al. Taxol biosynthesis: differential transformations of taxadien-5 alpha-ol and its acetate ester by cytochrome P450 hydroxylases from Taxus suspension cells. Arch. Biochem. Biophys. 390, 265–278 (2001).
Guo, J. et al. CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts. Proc. Natl. Acad. Sci. USA 110, 12108–12113 (2013).
Zhou, Y.J. et al. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. J. Am. Chem. Soc. 134, 3234–3241 (2012).
Hom, E.F. & Murray, A.W. Niche engineering demonstrates a latent capacity for fungal-algal mutualism. Science 345, 94–98 (2014).
Pillai, V.C., Snyder, R.O., Gumaste, U., Thekkumkara, T.J. & Mehvar, R. Effects of transient overexpression or knockdown of cytochrome P450 reductase on reactive oxygen species generation and hypoxia reoxygenation injury in liver cells. Clin. Exp. Pharmacol. Physiol. 38, 846–853 (2011).
Reed, J.R., Cawley, G.F. & Backes, W.L. Inhibition of cytochrome P450 1A2-mediated metabolism and production of reactive oxygen species by heme oxygenase-1 in rat liver microsomes. Drug Metab. Lett. 5, 6–16 (2011).
Artsatbanov, V.Y. et al. Influence of oxidative and nitrosative stress on accumulation of diphosphate intermediates of the non-mevalonate pathway of isoprenoid biosynthesis in corynebacteria and mycobacteria. Biochemistry 77, 362–371 (2012).
Rontein, D. et al. CYP725A4 from yew catalyzes complex structural rearrangement of taxa-4(5),11(12)-diene into the cyclic ether 5(12)-oxa-3(11)-cyclotaxane. J. Biol. Chem. 283, 6067–6075 (2008).
Xue, J. & Ahring, B.K. Enhancing isoprene production by genetic modification of the 1-deoxy-d-xylulose-5-phosphate pathway in Bacillus subtilis. Appl. Environ. Microbiol. 77, 2399–2405 (2011).
Zhou, K., Zou, R., Zhang, C., Stephanopoulos, G. & Too, H.P. Optimization of amorphadiene synthesis in Bacillus subtilis via transcriptional, translational, and media modulation. Biotechnol. Bioeng. 110, 2556–2561 (2013).
Doshi, R., Nguyen, T. & Chang, G. Transporter-mediated biofuel secretion. Proc. Natl. Acad. Sci. USA 110, 7642–7647 (2013).
Santos, C.N., Xiao, W. & Stephanopoulos, G. Rational, combinatorial, and genomic approaches for engineering l-tyrosine production in Escherichia coli. Proc. Natl. Acad. Sci. USA 109, 13538–13543 (2012).
Minami, H. et al. Microbial production of plant benzylisoquinoline alkaloids. Proc. Natl. Acad. Sci. USA 105, 7393–7398 (2008).
Choi, Y.J. & Lee, S.Y. Microbial production of short-chain alkanes. Nature 502, 571–574 (2013).
Leber, C. & Da Silva, N.A. Engineering of Saccharomyces cerevisiae for the synthesis of short chain fatty acids. Biotechnol. Bioeng. 111, 347–358 (2014).
Craft, D.L., Madduri, K.M., Eshoo, M. & Wilson, C.R. Identification and characterization of the CYP52 family of Candida tropicalis ATCC 20336, important for the conversion of fatty acids and alkanes to alpha,omega-dicarboxylic acids. Appl. Environ. Microbiol. 69, 5983–5991 (2003).
Zou, R., Zhou, K., Stephanopoulos, G. & Too, H.P. Combinatorial engineering of 1-deoxy-d-xylulose 5-phosphate pathway using cross-lapping in vitro assembly (CLIVA) method. PLoS ONE 8, e79557 (2013).
Flagfeldt, D.B., Siewers, V., Huang, L. & Nielsen, J. Characterization of chromosomal integration sites for heterologous gene expression in Saccharomyces cerevisiae. Yeast 26, 545–551 (2009).
Avalos, J.L., Fink, G.R. & Stephanopoulos, G. Compartmentalization of metabolic pathways in yeast mitochondria improves the production of branched-chain alcohols. Nat. Biotechnol. 31, 335–341 (2013).
Acknowledgements
We acknowledge useful discussions and input from A. Ghaderi, F. Lam, H. Zhang, J. Avalos and W. Wang. This work was supported by National Institutes of Health grant 1-R01-GM085323-01A1 and the Singapore MIT Alliance.
Author information
Authors and Affiliations
Contributions
K.Z. and G.S. conceived the project. K.Z., K.Q., S.E. and G.S. designed the experiments, analyzed the results and wrote the manuscript. K.Z., K.Q. and S.E. did all the experiments.
Corresponding author
Ethics declarations
Competing interests
The authors have filed a patent on the co-culture concept.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–19 and Supplementary Tables 1–4 (PDF 1669 kb)
Rights and permissions
About this article
Cite this article
Zhou, K., Qiao, K., Edgar, S. et al. Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol 33, 377–383 (2015). https://doi.org/10.1038/nbt.3095
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3095
This article is cited by
-
Compositional and temporal division of labor modulates mixed sugar fermentation by an engineered yeast consortium
Nature Communications (2024)
-
Cross-feeding promotes heterogeneity within yeast cell populations
Nature Communications (2024)
-
A molecular toolkit of cross-feeding strains for engineering synthetic yeast communities
Nature Microbiology (2024)
-
Spontaneously established syntrophic yeast communities improve bioproduction
Nature Chemical Biology (2023)
-
Eco-evolutionary modelling of microbial syntrophy indicates the robustness of cross-feeding over cross-facilitation
Scientific Reports (2023)