Abstract
A large amount of radionuclides was released from the Fukushima Dai-ichi Nuclear Power Station (FDNPS) following the damage caused by the tsunami due to the Great East Japan Earthquake on 11 March 2011. Although many radionuclides in various environmental samples around the FDNPS have been measured, 3H in the terrestrial environment has not yet been reported. We present here the first survey results of 3H concentrations in plant samples collected around the FDNPS in 2011 from shortly after the accident. The free-water 3H concentrations in herbaceous plant shoots and evergreen tree leaves were considerably higher than the previous background concentration and diminished with distance from the FDNPS. Although reconstruction of atmospheric 3H concentrations after the accident is difficult, a rough estimate of the radiation dose due to 3H inhalation about 20 km from the FDNPS is on the order of a few microsieverts (μSv).
Similar content being viewed by others
Introduction
A large amount of radionuclides was released from the Fukushima Dai-ichi Nuclear Power Station (FDNPS) following the damage caused by the tsunami due to the Great East Japan Earthquake on 11 March 20111,2,3. Tritium (3H, or T) is generated in nuclear power plants by various nuclear reactions and is present as tritiated water (HTO), tritiated hydrogen gas (HT) and other chemical forms in nuclear fuel rods4. When containment of radionuclides in nuclear fuel rods fails, HTO and HT are released into the air with other volatile radionuclides such as radiokrypton, radioxenon and radioiodine. Although many radionuclides, including 131I and 134,137Cs, in various environmental samples around the power station have been measured5,6, the only study of 3H so far published reports a survey of river water samples collected in November–December 2011, which found no clear effect of FDNPS7. We collected plant samples in 2011, including samples taken just after the accident and measured the free-water tritium (FWT) concentration. The results are discussed in relation to atmospheric HTO concentration.
Results
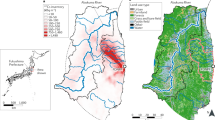
We collected samples from flower beds along sidewalks and green verges alongside roads outside the 20-km evacuation zone on 17–19 March, 12–14 April and 26 April 2011. The measured FWT concentrations were generally higher than the background 3H concentration (e.g. <1.5 Bq L−1 in atmospheric moisture samples in Fukushima Prefecture during 2008–20108) (Fig. 1a, b). The FWT concentrations roughly depended on the distance from the FDNPS; the maximum FWT concentration was 167 Bq/L in an unidentified herbaceous plant 20 km north-west of FDNPS (L13 in Fig. 1b). The FWT concentrations in the samples collected in April were lower than those collected in March at two resampled points (L01 vs L26/27; L09 vs L22/23; Fig. 1a, b) and at nearby sampling points (L02/L03 vs L29; L04 vs L24/L25; Fig. 1a, b).
Further samples were collected both outside and inside the 20-km evacuation zone on 21 and 28–29 July and on 1–5 and 9–11 August. The samples outside the zone were collected as before and those inside it were taken from paddy fields in fallow. The FWT concentrations in these samples were generally lower than the earlier ones (Fig. 1c).
Discussion
Atmospheric HTO concentrations in Fukushima Prefecture were not reported after the accident. Our plant data allow the concentrations to be reconstructed. The free water within plant shoots is supplied from the soil via the roots, from the air mainly via the stomata and by the oxidation of organic material9. Shortly after the accident, when the radioactive plume was spreading around the damaged reactor, the uptake of HTO vapour from the atmosphere through the leaves was the main route of entry into plants. After the plume had diffused, the HTO deposited in the soil became the main source of HTO in plants.
Belot et al. reported that an equilibrium between the FWT concentration in grape plants (FWTplant) and the atmospheric HTO concentration (HTOair) was established within a few hours10. If the soil moisture contains no HTO, then FWTplant/HTOair approximates the relative humidity (RH) in air10. The average RH during 17–19 March and 12–26 April in Fukushima City was 56% during each period, when the average air temperatures were 3 and 11°C, respectively11. So we assume that FWTplant/HTOair ≈ 0.5. HTOair can thus be estimated from that ratio. We also assume that a short-term peak HTOair will not affect FWTplant.
The rates of downward migration of water measured in surface soil in upland fields in Japan were 80–120 cm/y12,13,14. Therefore, the HTO assumed to have been deposited on 15 March, when the rate of release of radionuclides from FDNPS was maximum15, would have reached 7–10 cm deep in April and our plant samples in April would have absorbed deposited HTO via root. As described before, the free water within plant shoots is a mixture of soil water, atmospheric moisture and oxidation water of organic material. When HTOair concentration is estimated from FWTplant, neglecting contribution of soil water and the oxidation water of organic material leads to overestimated contribution of atmospheric moisture and consequently to conservative HTOair concentration. So we have assumed the FWTplant/HTOair ratio of 0.5 as the maximum value during April.
The FWT concentrations in the plant samples collected in April (L22, 23, 24, 25, 26, 27, 29) were 9±5% (6–38% in range) of those collected in March at the same and nearby points (L01, 02, 03, 04, 09) (Supp. Table 1). Applying the minimum value (6%) to the maximum FWT concentration in the unidentified herbaceous plant (167 Bq L−1 at L13) gives FWT ~2.8 kBq L−1 on 17 March. That value gives an HTO concentration in atmospheric moisture of 5.6 kBq L−1. We conservatively assume that the atmospheric HTO concentration remained high until the end of July. From our assumed values of absolute humidity (calculated from monthly mean RH and air temperature in Fukushima City11), breathing rate (20 m3/d) and dose conversion factor (ICRP-7216), we estimate the committed effective dose (CED) from HTO inhalation to be 3 μSv.
The CED at F17 (1 km from L13) from August to December was estimated to be only 10 nSv. On 10 August 2011, when the RH was 65%, the HTO concentration in atmospheric moisture was estimated to be only 17 Bq L−1.
The CED from inhalation of atmospheric HTO during 2011 was roughly and conservatively estimated to be 3 μSv just outside the 20-km evacuation zone. To estimate the dose inside the zone requires further study of the behaviour of 3H in the environment and modelling of 3H diffusion from the FDNPS.
Methods
Plant samples were collected during five sampling campaigns in 2011: 17–19 March, 12–14 April, 26 April, 21 and 28–29 July and 1–5 and 9–11 August. Herbaceous plant shoots and evergreen tree leaves were collected at sampling points >20 km from FDNPS (Fig. 1a, b) in March and April (Supp. Table 1). Herbaceous plant shoots were collected at sampling points both <20 km and >20 km from FDNPS (Fig. 1c) in July and August (Supp. Table 2).
Free water in the samples was absorbed into silica gel (Wako, Tokyo), which had been dried at 150°C for 24 h before use, at room temperature for >2 weeks in a water-vapour-tight laminate bag (AL-30L, Seisannipponsha, Tokyo) and recovered by heating of the silica gel at 250°C for 6 h. Since part of the crystalline water in the silica gel was also released, the volume of crystalline water recovered was separately measured by an isotope dilution method using HTO at a known concentration. The concentration of 3H in the crystalline water was separately determined by mass spectrometry17 of 3He (the decay product of 3H) from a silica gel sample sealed in an aluminosilicate glass vessel and confirmed to be negligible. To verify the pretreatment method, we compared the 3H concentration obtained by using silica gel with that obtained by lyophilization of four plant samples. The results agreed very well.
The concentration of 3H in the water samples was measured by liquid scintillation counter (LB-7, Hitachi Aloka Medical, Tokyo) calibrated with the NIST standard SRM 4361C.
References
Nuclear Emergency Response Headquarters, Government of Japan. Report of the Japanese Government to the IAEA Ministerial Conference on Nuclear Safety. Available at http://www.iaea.org/newscenter/focus/fukushima/japan-report/ (2011).
Brumfiel, G. & Cyranoski, D. Quake sparks nuclear crisis. Nature 471, 273–275 (2011).
Butler, C. Radioactivity spreads in Japan. Nature 471, 555–556 (2011).
National Council on Radiation Protection. Tritium in the environment. Report No. 62. NCRP, Washington, DC (1979).
Ministry of Education, Culture, Sports, Science & Technology of Japan. Monitoring information of environmental radioactivity level. Available at http://radioactivity.mext.go.jp/en/ (2012).
Emergency Operation Center, Ministry of Education, Culture, Sports, Science and Technology; Agriculture, Forestry and Fisheries Research Council, Ministry of Agriculture, Forestry and Fisheries. Summary of Results of the Research on Distribution of Radioactive Substances Discharged by the Accident at TEPCO's Fukushima Dai-ichi NPP. Available at http://radioactivity.mext.go.jp/en/contents/1000/294/view.html (2012).
Nuclear Emergency Response Headquarters (Radioactivity Team), Disaster Provision Main Office of Fukushima Prefecture (Nuclear Power Team). Readings of Environmental Radiation Monitoring of River Water, etc. (Tritium). Available at http://radioactivity.mext.go.jp/en/contents/6000/5268/view.html (2012).
Ministry of Education, Culture, Sports, Science and Technology. Environmental Radioactivity and Radiation in Japan. Available at http://www.kankyo-hoshano.go.jp (in Japanese) (2012).
Boyer, C. et al. Tritium in plants: a review of current knowledge. Env Exp Bot 67 (1), 34–51 (2009).
Belot, Y., Gauthier, D., Camus, H. & Caput, C. Prediction of the flux of tritiated water from air to plant leaves. Health Phys. 37 (4), 575–583 (1979).
Japan Meteorological Agency. Available at http://www.data.jma.go.jp (in Japanese).
Igarashi, T. Doctoral dissertation, Hokkaido University. Available at http://hdl.handle.net/2115/20145 (in Japanese) (2012).
Yuita, K. Movement of iodine and rain water from the atmosphere to the plant soil–water system by the activable tracer technique. In Proc 22 Jpn Conf on Radiation and Radioisotopes. B230 (Japan Atomic industrial Forum, 1996). (in Japanese with English abstract).
Kaneko, F. et al. The conditions of leaching of soil water and nitrate nitrogen originating from the fertilizer in the field where Kanto loam deposited by using of the pan lysimeter method. Jpn J Soil Sci Plant Nutr 73, 501–507 (2002) (in Japanese with English abstract).
Chino, M. et al. Preliminary estimation of release amounts of 131I and 137Cs accidentally discharged from the Fukushima Daiichi Nuclear Power Plant into the atmosphere. J Nucl Sci Technol 48 (7), 1129–1134 (2011).
International Commission on Radiological Protection. Age-dependent doses to the members of the public from intake of radionuclides – Part 5, Compilation of ingestion and inhalation coefficients. Ann Int Comm Radiol Prot 26, 84 (1996).
Kakiuchi, H. et al. Low-level measurement with a noble gas mass spectrometer for organically bound tritium in environmental samples. Fusion Sci Technol 60 (4), 1256–1259 (2011).
Acknowledgements
We are grateful to Fukushima Agricultural Technology Centre, Japan for collecting samples. This work was performed under contract with the government of Aomori Prefecture, Japan.
Author information
Authors and Affiliations
Contributions
H.K. designed the study, carried out sampling and analysis. N.A., H.H., S.U., S.T., M.Y., M.H., A.S. and H.T. contributed to sampling. K.N. identified plant samples. S.H. interpreted the data and wrote the manuscript text. All authors discussed the results and commented on the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Kakiuchi, H., Akata, N., Hasegawa, H. et al. Concentration of 3H in plants around Fukushima Dai-ichi Nuclear Power Station. Sci Rep 2, 947 (2012). https://doi.org/10.1038/srep00947
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep00947
This article is cited by
-
Temporal variation of tritium concentration in monthly precipitation collected at a Difficult-to-Return Zone in Namie Town, Fukushima Prefecture, Japan
Environmental Science and Pollution Research (2024)
-
Record of 3H and 36Cl from the Fukushima nuclear accident recovered from soil water in the unsaturated zone at Koriyama
Scientific Reports (2023)
-
Levels and behavior of environmental tritium in East Asia
Nuclear Science and Techniques (2022)
-
Characterization of atmospheric tritiated water concentration in the vicinity of the fukushima daiichi nuclear power plant
Journal of Radioanalytical and Nuclear Chemistry (2022)
-
Cryogenic vacuum extraction scarcely changes low-level tritium (3H) concentrations in free water extracted from environmental samples
Journal of Radioanalytical and Nuclear Chemistry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.