Abstract
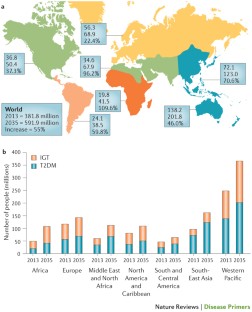
Type 2 diabetes mellitus (T2DM) is an expanding global health problem, closely linked to the epidemic of obesity. Individuals with T2DM are at high risk for both microvascular complications (including retinopathy, nephropathy and neuropathy) and macrovascular complications (such as cardiovascular comorbidities), owing to hyperglycaemia and individual components of the insulin resistance (metabolic) syndrome. Environmental factors (for example, obesity, an unhealthy diet and physical inactivity) and genetic factors contribute to the multiple pathophysiological disturbances that are responsible for impaired glucose homeostasis in T2DM. Insulin resistance and impaired insulin secretion remain the core defects in T2DM, but at least six other pathophysiological abnormalities contribute to the dysregulation of glucose metabolism. The multiple pathogenetic disturbances present in T2DM dictate that multiple antidiabetic agents, used in combination, will be required to maintain normoglycaemia. The treatment must not only be effective and safe but also improve the quality of life. Several novel medications are in development, but the greatest need is for agents that enhance insulin sensitivity, halt the progressive pancreatic β-cell failure that is characteristic of T2DM and prevent or reverse the microvascular complications. For an illustrated summary of this Primer, visit: http://go.nature.com/V2eGfN
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
DeFronzo, R. A. Banting lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58, 773–795 (2009). A classic review of the aetiology of T2DM, with a therapeutic approach based on its pathophysiology.
Abdul-Ghani, M. A., Tripathy, D. & DeFronzo, R. A. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29, 1130–1139 (2006).
Gerstein, H. C. et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res. Clin. Pract. 78, 305–312 (2007).
Hawa, M. I. et al. Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: action LADA 7. Diabetes Care 36, 908–913 (2013).
Gardner, D. S. & Tai, E. S. Clinical features and treatment of maturity onset diabetes of the young (MODY). Diabetes. Metab. Syndr. Obes. 5, 101–108 (2012).
American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 37, S14–S80 (2014). A comprehensive overview of the standards of medical care published by the ADA.
DeFronzo, R. A. & Abdul-Ghani, M. A. Preservation of β-cell function: the key to diabetes prevention. J. Clin. Endocrinol. Metab. 96, 2354–2366 (2011).
Ferrannini, E., Gastaldelli, A. & Iozzo, P. Pathophysiology of prediabetes. Med. Clin. North Am. 95, 327–339 (2011).
Garvey, W. T. et al. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care 37, 912–921 (2014).
Nathan, D. M. et al. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30, 753–759 (2007).
DeFronzo, R. A. et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N. Engl. J. Med. 364, 1104–1115 (2011). A large prospective study demonstrating the efficacy of thiazolidinediones in preventing the progression of IGT to T2DM.
Zinman, B. et al. Low-dose combination therapy with rosiglitazone and metformin to prevent type 2 diabetes mellitus (CANOE trial): a double-blind randomised controlled study. Lancet 376, 103–111 (2010).
Dansinger, M. L., Tatsioni, A., Wong, J. B., Chung, M. & Balk, E. M. Meta-analysis: the effect of dietary counseling for weight loss. Ann. Intern. Med. 147, 41–50 (2007).
Purcell, K. et al. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol. 2, 954–962 (2014).
Ali, M. K., Echouffo-Tcheugui, J. & Williamson, D. F. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff. (Millwood) 31, 67–75 (2012).
Tuomilehto, J. et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 344, 1343–1350 (2001).
Inzucchi, S. E. et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35, 1364–1379 (2012). ADA position statement on the treatment of T2DM, advocating a stepped care approach starting with metformin.
American Association of Clinical Endocrinologists. AACE Comprehensive Diabetes Algorithm 2013 Consensus Statement. Endocr. Pract. Suppl. 1, 1–87 (2015). AACE position statement on the treatment of T2DM, advocating initial monotherapy or combination therapy based upon the starting HbA1c, and recommending various antidiabetic medications as initial therapy.
Pozzilli, P. et al. The A1C and ABCD of glycaemia management in type 2 diabetes: a physician's personalized approach. Diabetes Metab. Res. Rev. 26, 239–244 (2010). The first published report by key opinion leaders recommending individualized therapy based on the age and body weight of patients, the presence or absence of complications, and duration and aetiology of disease.
International Diabetes Federation. IDF Diabetes Atlas 6th Edition. IDF[online], (2013).
Hu, F. B. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34, 1249–1257 (2011). An important study emphasizing the role of diet, physical activity and genes — beyond obesity — in the diabetes epidemic that is engulfing Asian countries as they are exposed to westernization.
Chan, J. C. et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 301, 2129–2140 (2009).
Ley, S. H., Hamdy, O., Mohan, V. & Hu, F. B. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 383, 1999–2007 (2014).
Grøntved, A., Rimm, E. B., Willett, W. C., Andersen, L. B. & Hu, F. B. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch. Intern. Med. 172, 1306–1312 (2012).
Grøntved, A. & Hu, F. B. Television viewing and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a meta-analysis. JAMA 305, 2448–2455 (2011).
Cappuccio, F. P., D'Elia, L., Strazzullo, P. & Miller, M. A. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 33, 414–420 (2009).
Pan, A., Schernhammer, E. S., Sun, Q. & Hu, F. B. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 8, e1001141 (2011).
Barnett, A. H., Eff, C., Leslie, R. D. & Pyke, D. A. Diabetes in identical twins. A study of 200 pairs. Diabetologia 20, 87–93 (1981).
Wang, Y. C., McPherson, K., Marsh, T., Gortmaker, S. L. & Brown, M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378, 815–825 (2011).
Wang, X. et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 36, 166–175 (2013).
Li, S., Shin, H. J., Ding, E. L. & van Dam, R. M. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302, 179–188 (2009).
Ding, E. L. et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 361, 1152–1163 (2009).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nat. Med. 17, 448–453 (2011).
Esteve, E., Ricart, W. & Fernández-Real, J.-M. Gut microbiota interactions with obesity, insulin resistance and type 2 diabetes: did gut microbiote co-evolve with insulin resistance? Curr. Opin. Clin. Nutr. Metab. Care 14, 483–490 (2011).
Hu, F. B. et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 345, 790–797 (2001).
Schellenberg, E. S., Dryden, D. M., Vandermeer, B., Ha, C. & Korownyk, C. Lifestyle interventions for patients with and at risk for type 2 diabetes. Ann. Intern. Med. 159, 543–551 (2013). A comprehensive review of the effectiveness of lifestyle intervention in the treatment of T2DM, emphasizing that, although initially successful, most subjects with diabetes regain the majority of lost weight over the subsequent 3–5 years.
DeFronzo, R. A. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 53, 1270–1287 (2010). A comprehensive review describing the role of excess tissue lipid deposition in the development of insulin resistance, β-cell failure and atherosclerotic cardiovascular disease: that is, lipotoxicity.
Hemminki, K., Li, X., Sundquist, K. & Sundquist, J. Familial risks for type 2 diabetes in Sweden. Diabetes Care 33, 293–297 (2010).
Groop, L. et al. Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes 45, 1585–1593 (1996).
Lyssenko, V. et al. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 54, 166–174 (2005).
Grant, S. F. et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 38, 320–323 (2006).
Lyssenko, V. et al. Mechanisms by which common variants in the TCF7L2 gene increase risk of type 2 diabetes. J. Clin. Invest. 117, 2155–2163 (2007).
Sladek, R. et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445, 881–885 (2007).
Saxena, R. et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316, 1331–1336 (2007).
Morris, A. P. et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat. Genet. 44, 981–990 (2012).
Flannick, J. et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat. Genet. 46, 357–363 (2014).
Lyssenko, V. et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat. Genet. 41, 82–88 (2009).
Rosengren, A. H. et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science 327, 217–220 (2010).
Tang, Y. et al. Genotype-based treatment of type 2 diabetes with an α2A-adrenergic receptor antagonist. Sci. Transl Med. 6, 257ra139 (2014). These paper provides an example in which a genetic finding in an animal model of diabetes has been translated into a drug target in humans, the ADRA2A gene.
De Jesus, D. F. & Kulkarni, R. N. Epigenetic modifiers of islet function and mass. Trends Endocrinol. Metab. 25, 628–636 (2014).
Ozcan, S. Minireview: microRNA function in pancreatic β cells. Mol. Endocrinol. 28, 1922–1933 (2014).
Lyssenko, V. et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N. Engl. J. Med. 359, 2220–2232 (2008). This paper presents a genetic explanation for the development of T2DM.
Travers, M. E. et al. Insights into the molecular mechanism for type 2 diabetes susceptibility at the KCNQ1 locus from temporal changes in imprinting status in human islets. Diabetes 62, 987–992 (2013).
Gulli, G., Ferrannini, E., Stern, M., Haffner, S. & DeFronzo, R. A. The metabolic profile of NIDDM is fully established in glucose-tolerant offspring of two Mexican-American NIDDM parents. Diabetes 41, 1575–1586 (1992).
Martin, B. C. et al. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340, 925–929 (1992).
Ferrannini, E. & Mari, A. β-cell function in type 2 diabetes. Metabolism 63, 1217–1227 (2014).
Kahn, S. E., Cooper, M. E. & Del Prato, S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383, 1068–1083 (2014).
Muller, D. C., Elahi, D., Tobin, J. D. & Andres, R. Insulin response during the oral glucose tolerance test: the role of age, sex, body fat and the pattern of fat distribution. Aging (Milano) 8, 13–21 (1996).
Nauck, M. A., Vardarli, I., Deacon, C. F., Holst, J. J. & Meier, J. J. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia 54, 10–18 (2011).
Madsbad, S. The role of glucagon-like peptide-1 impairment in obesity and potential therapeutic implications. Diabetes Obes. Metab. 16, 9–21 (2014).
Bays, H., Mandarino, L. & DeFronzo, R. A. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J. Clin. Endocrinol. Metab. 89, 463–478 (2004).
Perry, R. J., Samuel, V. T., Petersen, K. F. & Shulman, G. I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 510, 84–91 (2014). An excellent review of the specific lipid varieties and the molecular events through which they cause insulin resistance in the liver.
Bensellam, M., Laybutt, D. R. & Jonas, J.-C. The molecular mechanisms of pancreatic β-cell glucotoxicity: recent findings and future research directions. Mol. Cell. Endocrinol. 364, 1–27 (2012).
Ritzel, R. A., Meier, J. J., Lin, C.-Y., Veldhuis, J. D. & Butler, P. C. Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes 56, 65–71 (2007).
Collins, S., Pi, J. & Yehuda-Shnaidman, E. Uncoupling and reactive oxygen species (ROS) — a double-edged sword for β-cell function? “Moderation in all things”. Best Pract. Res. Clin. Endocrinol. Metab. 26, 753–758 (2012).
Cabrera, O. et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc. Natl Acad. Sci. USA 103, 2334–2339 (2006).
Hodson, D. J. et al. Lipotoxicity disrupts incretin-regulated human β cell connectivity. J. Clin. Invest. 123, 4182–4194 (2013).
Brandhorst, H., Brandhorst, D., Brendel, M. D., Hering, B. J. & Bretzel, R. G. Assessment of intracellular insulin content during all steps of human islet isolation procedure. Cell Transplant. 7, 489–495 (1998).
Rahier, J., Guiot, Y., Goebbels, R. M., Sempoux, C. & Henquin, J. C. Pancreatic β-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 10 (Suppl. 4), 32–42 (2008). A post-mortem study demonstrating a decline in β-cell mass with preservation of α-cell mass in individuals with T2DM.
Marselli, L. et al. Are we overestimating the loss of beta cells in type 2 diabetes? Diabetologia 57, 362–365 (2014).
Marchetti, P. et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 50, 2486–2494 (2007).
Marchetti, P. & Masini, M. Autophagy and the pancreatic beta-cell in human type 2 diabetes. Autophagy 5, 1055–1056 (2009).
Gupta, D. & Leahy, J. L. Islet amyloid and type 2 diabetes: overproduction or inadequate clearance and detoxification? J. Clin. Invest. 124, 3292–3294 (2014).
Mezza, T. et al. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes 63, 994–1007 (2014). This work in human islets describes the impact of insulin resistance on the relative proportion of α-cells and β-cells, and the functional consequences — in terms of insulin and glucagon secretion — of this chronic adaptation.
Deng, S. et al. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 53, 624–632 (2004).
Igoillo-Esteve, M. et al. Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia 53, 1395–1405 (2010).
Giacca, A., Xiao, C., Oprescu, A. I., Carpentier, A. C. & Lewis, G. F. Lipid-induced pancreatic β-cell dysfunction: focus on in vivo studies. Am. J. Physiol. Endocrinol. Metab. 300, E255–E262 (2010).
Halban, P. A. et al. β-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. J. Clin. Endocrinol. Metab. 99, 1983–1992 (2014).
Ferrannini, E. et al. Natural history and physiological determinants of changes in glucose tolerance in a non-diabetic population: the RISC Study. Diabetologia 54, 1507–1516 (2011). This longitudinal study of non-diabetic subjects identifies baseline insulin resistance and β-cell dysfunction as predictors of future dysglycaemia.
Michaliszyn, S. F. et al. β-cell function, incretin effect, and incretin hormones in obese youth along the span of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes 63, 3846–3855 (2014).
Mari, A. et al. Mechanisms of the incretin effect in subjects with normal glucose tolerance and patients with type 2 diabetes. PLoS ONE 8, e73154 (2013).
Holst, J. J., Knop, F. K., Vilsbøll, T., Krarup, T. & Madsbad, S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care 34, S251–S257 (2011).
Camastra, S. et al. Long-term effects of bariatric surgery on meal disposal and β-cell function in diabetic and nondiabetic patients. Diabetes 62, 3709–3717 (2013).
Ferrannini, E. The stunned β cell: a brief history. Cell Metab. 11, 349–352 (2010).
Shulman, G. I. et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N. Engl. J. Med. 322, 223–228 (1990). This study demonstrated that defects in insulin-stimulated muscle glycogen synthesis was the major factor responsible for whole-body insulin resistance in patients with T2DM.
Groop, L. C. et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Invest. 84, 205–213 (1989).
Guilherme, A., Virbasius, J. V., Puri, V. & Czech, M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 367–377 (2008).
Gerich, J. E., Meyer, C., Woerle, H. J. & Stumvoll, M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24, 382–391 (2001).
Honka, H. et al. Validation of [18F]fluorodeoxyglucose and positron emission tomography (PET) for the measurement of intestinal metabolism in pigs, and evidence of intestinal insulin resistance in patients with morbid obesity. Diabetologia 56, 893–900 (2013).
Meijer, R. I. et al. Insulin-induced microvascular recruitment in skin and muscle are related and both are associated with whole-body glucose uptake. Microcirculation 19, 494–500 (2012).
Blázquez, E., Velázquez, E., Hurtado-Carneiro, V. & Ruiz-Albusac, J. M. Insulin in the brain: its pathophysiological implications for states related with central insulin resistance, type 2 diabetes and Alzheimer's disease. Front. Endocrinol. (Lausanne) 5, 161 (2014).
Kleinridders, A., Ferris, H. A., Cai, W. & Kahn, C. R. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63, 2232–2243 (2014).
Kulkarni, R. N. et al. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96, 329–339 (1999). An insightful study documenting that β-cell-specific insulin receptor knockout results in markedly impaired insulin secretion and overt diabetes, thereby providing a unifying mechanism whereby insulin resistance explains both the defects in insulin-stimulated tissue glucose uptake and decreased insulin secretion.
Oliveira, J. M., Rebuffat, S. A., Gasa, R. & Gomis, R. Targeting type 2 diabetes: lessons from a knockout model of insulin receptor substrate 2. Can. J. Physiol. Pharmacol. 92, 613–620 (2014).
Samuel, V. T. & Shulman, G. I. Mechanisms for insulin resistance: common threads and missing links. Cell 148, 852–871 (2012). An excellent review of the molecular mechanism responsible for insulin resistance in T2DM and obesity.
Magnusson, I., Rothman, D. L., Katz, L. D., Shulman, R. G. & Shulman, G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J. Clin. Invest. 90, 1323–1327 (1992). This study demonstrated that increased rates of hepatic glucose production in patients with poorly controlled T2DM could entirely be attributed to increased rates of gluconeogenesis.
Matsuda, M. et al. Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism 51, 1111–1119 (2002).
Samuel, V. T. et al. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with type 2 diabetes. Proc. Natl Acad. Sci. USA 106, 12121–12126 (2009).
Baron, A. D., Schaeffer, L., Shragg, P. & Kolterman, O. G. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36, 274–283 (1987).
DeFronzo, R. A., Ferrannini, E., Hendler, R., Wahren, J. & Felig, P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc. Natl Acad. Sci. USA 75, 5173–5177 (1978).
Ferrannini, E. et al. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 37, 79–85 (1988).
DeFronzo, R. A. et al. Characterization of renal glucose reabsorption in response to dapagliflozin in healthy subjects and subjects with type 2 diabetes. Diabetes Care 36, 3169–3176 (2013).
Barrett, E. J., Wang, H., Upchurch, C. T. & Liu, Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am. J. Physiol. Endocrinol. Metab. 301, E252–E263 (2011).
Baron, A. D. Hemodynamic actions of insulin. Am. J. Physiol. 267, E187–E202 (1994).
Krüger, M. et al. Dissection of the insulin signaling pathway via quantitative phosphoproteomics. Proc. Natl Acad. Sci. USA 105, 2451–2456 (2008).
Cusi, K. et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J. Clin. Invest. 105, 311–320 (2000). The first study in humans with T2DM to demonstrate impaired insulin signal transduction through the IRS1–PI3K pathway in muscle, with normal to increased insulin signalling through the MAPK pathway.
Krook, A. et al. Characterization of signal transduction and glucose transport in skeletal muscle from type 2 diabetic patients. Diabetes 49, 284–292 (2000).
Copps, K. D. & White, M. F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55, 2565–2582 (2012).
Bouzakri, K. et al. IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes 55, 785–791 (2006).
Hiratani, K. et al. Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS-1. Biochem. Biophys. Res. Commun. 335, 836–842 (2005).
Krssak, M. et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42, 113–116 (1999).
Petersen, K. F. et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 109, 1345–1350 (2002).
Petersen, K. F. et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes 54, 603–608 (2005).
Lara-Castro, C. & Garvey, W. T. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol. Metab. Clin. North Am. 37, 841–856 (2008).
Yu, C. et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 277, 50230–50236 (2002).
Bezy, O. et al. PKCδ regulates hepatic insulin sensitivity and hepatosteatosis in mice and humans. J. Clin. Invest. 121, 2504–2517 (2011).
Samuel, V. T. et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 279, 32345–32353 (2004).
Samuel, V. T. et al. Inhibition of protein kinase Cε prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Invest. 117, 739–745 (2007).
Choi, C. S. et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 282, 22678–22688 (2007).
Morino, K. et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J. Clin. Invest. 115, 3587–3593 (2005).
Szendroedi, J. et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc. Natl Acad. Sci. USA 111, 9597–9602 (2014).
Larsen, P. J. & Tennagels, N. On ceramides, other sphingolipids and impaired glucose homeostasis. Mol. Metab. 3, 252–260 (2014).
Turpin, S. M. et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 (2014).
Cantley, J. L. et al. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc. Natl Acad. Sci. USA 110, 1869–1874 (2013).
Patti, M.-E. & Corvera, S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr. Rev. 31, 364–395 (2010). Mitochondrial dysfunction as a causative factor in the development of insulin resistance in T2DM is reviewed.
Ritov, V. B. et al. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54, 8–14 (2005).
Petersen, K. F. et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300, 1140–1142 (2003).
Petersen, K. F., Dufour, S., Befroy, D., Garcia, R. & Shulman, G. I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N. Engl. J. Med. 350, 664–671 (2004).
Mogensen, M. et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56, 1592–1599 (2007).
Petersen, K. F., Dufour, S. & Shulman, G. I. Decreased insulin-stimulated ATP synthesis and phosphate transport in muscle of insulin-resistant offspring of type 2 diabetic parents. PLoS Med. 2, e233 (2005).
Wang, C.-H., Wang, C.-C., Huang, H.-C. & Wei, Y.-H. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 280, 1039–1050 (2013).
Rains, J. L. & Jain, S. K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 50, 567–575 (2011).
Morino, K. et al. Regulation of mitochondrial biogenesis by lipoprotein lipase in muscle of insulin-resistant offspring of parents with type 2 diabetes. Diabetes 61, 877–887 (2012).
Romeo, G. R., Lee, J. & Shoelson, S. E. Metabolic syndrome, insulin resistance, and roles of inflammation — mechanisms and therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 32, 1771–1776 (2012).
Arkan, M. C. et al. IKK-β links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 (2005).
De Alvaro, C., Teruel, T., Hernandez, R. & Lorenzo, M. Tumor necrosis factor α produces insulin resistance in skeletal muscle by activation of inhibitor κB kinase in a p38 MAPK-dependent manner. J. Biol. Chem. 279, 17070–17078 (2004).
Howard, J. K. & Flier, J. S. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol. Metab. 17, 365–371 (2006).
Lebrun, P. & Van Obberghen, E. SOCS proteins causing trouble in insulin action. Acta Physiol. (Oxf.) 192, 29–36 (2008).
Uysal, K. T., Wiesbrock, S. M. & Hotamisligil, G. S. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-α-mediated insulin resistance in genetic obesity. Endocrinology 139, 4832–4838 (1998).
Ofei, F., Hurel, S., Newkirk, J., Sopwith, M. & Taylor, R. Effects of an engineered human anti-TNF-α antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes 45, 881–885 (1996).
Kim, J. K. et al. Prevention of fat-induced insulin resistance by salicylate. J. Clin. Invest. 108, 437–446 (2001).
Yuan, M. et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of IKKβ. Science 293, 1673–1677 (2001).
Goldfine, A. B. et al. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann. Intern. Med. 152, 346–357 (2010).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 (2011).
Nishimura, S. et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 15, 914–920 (2009).
Feuerer, M. et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15, 930–939 (2009).
Bertola, A. et al. Identification of adipose tissue dendritic cells correlated with obesity-associated insulin-resistance and inducing Th17 responses in mice and patients. Diabetes 61, 2238–2247 (2012).
Cai, D. et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med. 11, 183–190 (2005).
Perry, R. J. et al. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 (2015).
Mori, M. A. et al. A systems biology approach identifies inflammatory abnormalities between mouse strains prior to development of metabolic disease. Diabetes 59, 2960–2971 (2010).
Mauer, J. et al. Myeloid cell-restricted insulin receptor deficiency protects against obesity-induced inflammation and systemic insulin resistance. PLoS Genet. 6, e1000938 (2010).
Shi, H. et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 (2006).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Boden, G. et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57, 2438–2444 (2008).
Eizirik, D. L., Cardozo, A. K. & Cnop, M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 29, 42–61 (2008). A comprehensive review of ER stress and the UPR in the development of insulin resistance and obesity.
Gregor, M. F. et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58, 693–700 (2009).
Ozawa, K. et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 54, 657–663 (2005).
Herschkovitz, A. et al. Common inhibitory serine sites phosphorylated by IRS-1 kinases, triggered by insulin and inducers of insulin resistance. J. Biol. Chem. 282, 18018–18027 (2007).
Boden, G. Endoplasmic reticulum stress: another link between obesity and insulin resistance/inflammation? Diabetes 58, 518–519 (2009).
Sengupta, S., Peterson, T. R. & Sabatini, D. M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell 40, 310–322 (2010).
Shah, O. J., Wang, Z. & Hunter, T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 14, 1650–1656 (2004).
Ozcan, U. et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell 29, 541–551 (2008).
Park, S. W. et al. The regulatory subunits of PI3K, p85α and p85β, interact with XBP-1 and increase its nuclear translocation. Nat. Med. 16, 429–437 (2010).
Stratton, I. M. et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321, 405–412 (2000). A seminal UK Prospective Diabetes Study study unequivocally demonstrating that improved glycaemic control reduced the incidence of microvascular, and to a lesser extent, macrovascular complications in patients with T2DM.
Holman, R. R., Paul, S. K., Bethel, M. A., Matthews, D. R. & Neil, H. A. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 359, 1577–1589 (2008). A long-term follow-up of the UK Prospective Diabetes Study showing that early intensive glycaemic control has a persistent impact on preventing both microvascular and macrovascular complications long after initiation of the intensified antidiabetic regimen has been discontinued: that is, the ‘legacy effect’.
Brownlee, M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, 1615–1625 (2005). A lucid discussion of the molecular pathways involved in the development of diabetic microvascular complications.
Giacco, F. & Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 107, 1058–1070 (2010).
Coutinho, M., Gerstein, H. C., Wang, Y. & Yusuf, S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22, 233–240 (1999).
Taskinen, M.-R. & Borén, J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 239, 483–495 (2015). An up-to-date review of the pathogenesis of diabetic dyslipidaemia and its treatment.
Isomaa, B. et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24, 683–689 (2001).
Adler, A. I. et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 321, 412–419 (2000).
Williams, B. Treating hypertension in patients with diabetes: when to start and how low to go? JAMA 313, 573–574 (2015). The optimal blood pressure goal in hypertensive patients with T2DM is discussed in light of the controversial results observed in the blood pressure arm of the ACCORD trial.
Lastra, G., Syed, S., Kurukulasuriya, L. R., Manrique, C. & Sowers, J. R. Type 2 diabetes mellitus and hypertension: an update. Endocrinol. Metab. Clin. North Am. 43, 103–122 (2014).
International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 32, 1327–1334 (2009).
[No authors listed.] Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 20, 1183–1197 (1997). A reference publication by the ADA on the diagnosis and classification of diabetes mellitus.
Herman, W. H. Diabetes epidemiology: guiding clinical and public health practice: the Kelly West Award Lecture, 2006. Diabetes Care 30, 1912–1919 (2007). A landmark lecture providing a comprehensive overview of the epidemiology of T2DM and the public health implications for diabetes prevention.
DECODE Study Group, European Diabetes Epidemiology Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care 26, 688–696 (2003).
Engelgau, M. M., Narayan, K. M. & Herman, W. H. Screening for type 2 diabetes. Diabetes Care 23, 1563–1580 (2000).
LeFevre, M. L. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force Recommendation Statement. Ann. Intern. Med. 161, 587–593 (2014).
Lindström, J. & Tuomilehto, J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 26, 725–731 (2003).
Tabaei, B. P. & Herman, W. H. A multivariate logistic regression equation to screen for diabetes: development and validation. Diabetes Care 25, 1999–2003 (2002).
World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus (WHO, 1999).
Pan, X. R. et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT Diabetes Study. Diabetes Care 20, 537–544 (1997).
Knowler, W. C. et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346, 393–403 (2002).
Ramachandran, A. et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 49, 289–297 (2006).
Chiasson, J.-L. et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet 359, 2072–2077 (2002).
Kawamori, R. et al. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet 373, 1607–1614 (2009).
Knowler, W. C. et al. Prevention of type 2 diabetes with troglitazone in the Diabetes Prevention Program. Diabetes 54, 1150–1156 (2005).
Gerstein, H. C. et al. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368, 1096–1105 (2006).
Li, G. et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371, 1783–1789 (2008).
Lindström, J. et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet 368, 1673–1679 (2006).
Knowler, W. C. et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374, 1677–1686 (2009). Long-term follow-up of body weight regain and diabetes incidence in patients with IGT in the Diabetes Prevention Program treated with lifestyle heavy, lifestyle light and metformin, showing that gradual weight regain is the norm and that 40–50% of patients with IGT develop diabetes despite successful weight loss.
DeFronzo, R. A., Eldor, R. & Abdul-Ghani, M. Pathophysiologic approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care 36, S127–S138 (2013). A rational approach to the treatment of T2DM is presented based on its pathophysiology.
Raz, I. et al. Personalized management of hyperglycemia in type 2 diabetes: reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 36, 1779–1788 (2013).
Nakagami, T., Kawahara, R., Hori, S. & Omori, Y. Glycemic control and prevention of retinopathy in Japanese NIDDM patients. A 10-year follow-up study. Diabetes Care 20, 621–622 (1997).
Lim, E. L. et al. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 54, 2506–2514 (2011).
Jazet, I. M. et al. Loss of 50% of excess weight using a very low energy diet improves insulin-stimulated glucose disposal and skeletal muscle insulin signalling in obese insulin-treated type 2 diabetic patients. Diabetologia 51, 309–319 (2008).
Abdul-Ghani, M. A. et al. Initial combination therapy with metformin, pioglitazone and exenatide is more effective than sequential add-on therapy in subjects with new-onset diabetes. Results from the Efficacy and Durability of Initial Combination Therapy for Type 2 Diabetes (EDICT): a randomized trial. Diabetes Obes. Metab. 17, 268–275 (2015). This prospective randomized trial using a combination of antidiabetic agents proven to reverse known pathophysiological abnormalities in T2DM demonstrated superiority of glycaemic control compared with the stepped approach of metformin followed by a sulfonylurea and then basal insulin recommended by most national diabetes organizations.
Harrison, L. B., Adams-Huet, B., Raskin, P. & Lingvay, I. β-cell function preservation after 3.5 years of intensive diabetes therapy. Diabetes Care 35, 1406–1412 (2012).
Gram, J. et al. Pharmacological treatment of the pathogenetic defects in type 2 diabetes: the randomized multicenter South Danish Diabetes Study. Diabetes Care 34, 27–33 (2011).
DeFronzo, R. A. et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care 38, 384–393 (2015).
Weng, J. et al. Effect of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 371, 1753–1760 (2008).
Hu, Y. et al. Short-term intensive therapy in newly diagnosed type 2 diabetes partially restores both insulin sensitivity and β-cell function in subjects with long-term remission. Diabetes Care 34, 1848–1853 (2011). One of several recent studies demonstrating that intensive insulin therapy to correct the decompensated metabolic state in newly diagnosed patients with T2DM can lead to durable glycaemic control without or with a marked reduction in antidiabetic medications.
Xiang, A. H. et al. Effect of pioglitazone on pancreatic β-cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes 55, 517–522 (2006).
Astrup, A. et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. (Lond.) 36, 843–854 (2012).
Cusi, K., Consoli, A. & DeFronzo, R. A. Metabolic effects of metformin on glucose and lactate metabolism in noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 81, 4059–4067 (1996).
Turner, R. C., Cull, C. A., Frighi, V. & Holman, R. R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 281, 2005–2012 (1999). A landmark UK Prospective Diabetes Study documenting the need for progressive add-on therapies in newly diagnosed patients with T2DM receiving initial therapy with metformin or with a sulfonylurea.
Brown, J. B., Conner, C. & Nichols, G. A. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 33, 501–506 (2010).
Kahn, S. E. et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 355, 2427–2443 (2006). A 5-year ADOPT study demonstrating long-term durable HbA1c reduction with rosiglitazone compared with a progressive rise in HbA1c observed with metformin and sulfonylureas, and a more rapid deterioration of glycaemic control with sulfonylureas compared with metformin.
Madiraju, A. K. et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546 (2014).
Ferrannini, E. The target of metformin in type 2 diabetes. N. Engl. J. Med. 371, 1547–1548 (2014).
[No authors listed.] Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352, 854–865 (1998).
Maedler, K. et al. Sulfonylurea induced β-cell apoptosis in cultured human islets. J. Clin. Endocrinol. Metab. 90, 501–506 (2005).
Roumie, C. L. et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann. Intern. Med. 157, 601–610 (2012).
Simpson, S. H., Majumdar, S. R., Tsuyuki, R. T., Eurich, D. T. & Johnson, J. A. Dose–response relation between sulfonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. CMAJ 174, 169–174 (2006).
Simpson, S. H. et al. Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol. 3, 43–51 (2015). A review of the published literature that examines the relationship between sulfonylurea therapy and the development of adverse cardiovascular events.
Yki-Järvinen, H. Thiazolidinediones. N. Engl. J. Med. 351, 1106–1118 (2004).
Eldor, R., DeFronzo, R. A. & Abdul-Ghani, M. In vivo actions of peroxisome proliferator-activated receptors: glycemic control, insulin sensitivity, and insulin secretion. Diabetes Care 36, S162–S174 (2013). An exhaustive review of the mechanism of action, efficacy and side-effect profile of the thiazolidinedione class of antidiabetic medications.
Miyazaki, Y., He, H., Mandarino, L. J. & DeFronzo, R. A. Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes 52, 1943–1950 (2003).
Gastaldelli, A. et al. Thiazolidinediones improve β-cell function in type 2 diabetic patients. Am. J. Physiol. Endocrinol. Metab. 292, E871–E883 (2007).
DeFronzo, R. A. et al. Prevention of diabetes with pioglitazone in ACT NOW: physiologic correlates. Diabetes 62, 3920–3926 (2013).
Kahn, S. E. et al. Effects of rosiglitazone, glyburide, and metformin on β-cell function and insulin sensitivity in ADOPT. Diabetes 60, 1552–1560 (2011).
Dormandy, J. A. et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366, 1279–1289 (2005). A large prospective study (PROactive) demonstrating that pioglitazone significantly reduced the second principal end point of myocardial infarction, stroke and cardiovascular death; the primary end point did not reach statistical significance because of the inclusion of peripheral arterial disease and leg revascularization, which is known to be refractory to medical intervention, including statin therapy.
Aronoff, S. et al. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 23, 1605–1611 (2000).
Erdmann, E., Song, E., Spanheimer, R., van Troostenburg de Bruyn, A.-R. & Perez, A. Observational follow-up of the PROactive study: a 6-year update. Diabetes Obes. Metab. 16, 63–74 (2014).
[No authors listed.] Takeda announces completion of the post-marketing commitment to submit data to the FDA, the EMA and the PMDA for pioglitazone containing medicines including ACTOS. Takeda[online], (2014).
Levin, D. et al. Pioglitazone and bladder cancer risk: a multipopulation pooled, cumulative exposure analysis. Diabetologia 58, 493–504 (2015).
Kjems, L. L., Holst, J. J., Vølund, A. & Madsbad, S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on β-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 52, 380–386 (2003).
Vilsbøll, T., Krarup, T., Madsbad, S. & Holst, J. J. Defective amplification of the late phase insulin response to glucose by GIP in obese Type II diabetic patients. Diabetologia 45, 1111–1119 (2002).
Aroda, V. R. et al. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin. Ther. 34, 1247–1258.e22 (2012).
Deacon, C. F. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes. Metab. 13, 7–18 (2011).
Balas, B. et al. The dipeptidyl peptidase IV inhibitor vildagliptin suppresses endogenous glucose production and enhances islet function after single-dose administration in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 92, 1249–1255 (2007).
Drucker, D. J. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes 62, 3316–3323 (2013). A comprehensive review of the effect of incretin hormones on pancreatic hormone secretion and pathology by one of the world's leading authorities.
White, W. B. et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N. Engl. J. Med. 369, 1327–1335 (2013).
Scirica, B. M. et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N. Engl. J. Med. 369, 1317–1326 (2013).
Cervera, A. et al. Mechanism of action of exenatide to reduce postprandial hyperglycemia in type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 294, E846–E852 (2008).
Bunck, M. C. et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 34, 2041–2047 (2011). A landmark 3-year prospective study demonstrating the marked and durable improvement in β-cell function using the combined hyperglycaemic and euglycaemic insulin clamp techniques following exenatide treatment in patients with T2DM.
Klonoff, D. C. et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 24, 275–286 (2008).
Schwartz, S. & Kohl, B. A. Type 2 diabetes mellitus and the cardiometabolic syndrome: impact of incretin-based therapies. Diabetes Metab. Syndr. Obes. 3, 227–242 (2010).
Eng, C., Kramer, C. K., Zinman, B. & Retnakaran, R. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: a systematic review and meta-analysis. Lancet 384, 2228–2234 (2014).
Egan, A. G. et al. Pancreatic safety of incretin-based drugs — FDA and EMA assessment. N. Engl. J. Med. 370, 794–797 (2014).
Van de Laar, F. A. et al. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2, CD003639 (2005).
Esposito, K. et al. Dipeptidyl peptidase-4 inhibitors and HbA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 13, 594–603 (2011).
Richter, B., Bandeira-Echtler, E., Bergerhoff, K. & Lerch, C. L. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2, CD006739 (2008).
Abdul-Ghani, M. A., Norton, L. & DeFronzo, R. A. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr. Rev. 32, 515–531 (2011). An excellent review of the mechanism of action, efficacy and safety of the recently approved SGLT2 inhibitor class of antidiabetic medications.
Wright, E. M., Loo, D. D. & Hirayama, B. A. Biology of human sodium glucose transporters. Physiol. Rev. 91, 733–794 (2011).
Merovci, A. et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J. Clin. Invest. 124, 509–514 (2014).
Ferrannini, E. et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J. Clin. Invest. 124, 499–508 (2014).
Abdul-Ghani, M. A., DeFronzo, R. A. & Norton, L. Novel hypothesis to explain why SGLT2 inhibitors inhibit only 30–50% of filtered glucose load in humans. Diabetes 62, 3324–3328 (2013).
Cherney, D. Z. I. et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129, 587–597 (2014).
Holman, R. R. et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N. Engl. J. Med. 361, 1736–1747 (2009). A comparison of the efficacy and side-effect profile of commonly used complex insulin regimens for the treatment of patients with T2DM.
Gough, S. C. L. et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2, 885–893 (2014).
Wilding, J. P. et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann. Intern. Med. 156, 405–415 (2012).
Anderson, M., Powell, J., Campbell, K. M. & Taylor, J. R. Optimal management of type 2 diabetes in patients with increased risk of hypoglycemia. Diabetes Metab. Syndr. Obes. 7, 85–94 (2014).
Schopman, J. E. et al. The incidence of mild and severe hypoglycaemia in patients with type 2 diabetes mellitus treated with sulfonylureas: a systematic review and meta-analysis. Diabetes Metab. Res. Rev. 30, 11–22 (2014).
Desouza, C., Salazar, H., Cheong, B., Murgo, J. & Fonseca, V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care 26, 1485–1489 (2003).
Gerstein, H. C. et al. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559 (2008). The ORIGIN trial demonstrated that physiological insulin replacement doses (30–40 units per day) in newly diagnosed patients with T2DM could control HbA1c without an increased risk of cardiovascular events; however, the risk of hypoglycaemia was significantly increased, and the study did not examine the effect of higher doses of insulin, which are usually required to normalize glycaemia in more long-standing diabetes, on cardiovascular risk or other potential side effects of insulin therapy.
Cushman, W. C. et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N. Engl. J. Med. 362, 1575–1585 (2010).
James, P. A. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311, 507–520 (2014).
Emdin, C. et al. Association of cardiovascular trial registration with positive study findings: Epidemiological Study of Randomized Trials (ESORT). JAMA Intern. Med. 175, 304–307 (2015).
Testa, M. A. & Simonson, D. C. Health economic benefits and quality of life during improved glycemic control in patients with type 2 diabetes mellitus: a randomized, controlled, double-blind trial. JAMA 280, 1490–1496 (1998). This was the first randomized trial to demonstrate that better glucose control improves QOL, cognitive function and general perceived health, and reduces symptom distress and absenteeism from work.
Testa, M. A. & Simonson, D. C. Assesment of quality-of-life outcomes. N. Engl. J. Med. 334, 835–840 (1996).
Testa, M. A., Simonson, D. C. & Turner, R. R. Valuing quality of life and improvements in glycemic control in people with type 2 diabetes. Diabetes Care 21, C44–C52 (1998).
Bode, B. W. et al. Patient-reported outcomes following treatment with the human GLP-1 analogue liraglutide or glimepiride in monotherapy: results from a randomized controlled trial in patients with type 2 diabetes. Diabetes Obes. Metab. 12, 604–612 (2010).
Testa, M. A. et al. Comparative effectiveness of basal-bolus versus premix analog insulin on glycemic variability and patient-centered outcomes during insulin intensification in type 1 and type 2 diabetes: a randomized, controlled, crossover trial. J. Clin. Endocrinol. Metab. 97, 3504–3514 (2012). This randomized trial demonstrated that patient satisfaction with treatment was more positively affected by improved QOL, reduced glucose variability and better glycaemic control with a basal-bolus regimen than negatively affected by the burden of additional injections.
Cotter, A. P., Durant, N., Agne, A. A. & Cherrington, A. L. Internet interventions to support lifestyle modification for diabetes management: a systematic review of the evidence. J. Diabetes Complications 28, 243–251 (2014).
Rose, M. et al. The PROMIS Physical Function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J. Clin. Epidemiol. 67, 516–526 (2014).
DeFronzo, R. A. & Triplitt, C. Novel agents for T2DM. Diabetes Spectr. 27, 100–112 (2014). This article presents a more detailed review of novel antidiabetic agents that currently are being investigated in animals and humans for the treatment of T2DM.
Wong, A. K., Howie, J., Petrie, J. R. & Lang, C. C. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin. Sci. (Lond.) 116, 607–620 (2009).
Agrawal, N. K. & Kant, S. Targeting inflammation in diabetes: newer therapeutic options. World J. Diabetes 5, 697–710 (2014). Inflammation in insulin target tissues and β-cells is a now well-established pathogenetic abnormality T2DM. This article reviews the mechanism by which inflammation contributes to glucose intolerance in T2DM and potential interventions to suppress inflammation and improve insulin sensitivity and β-cell function.
Poy, M. N. et al. miR-375 maintains normal pancreatic α- and β-cell mass. Proc. Natl Acad. Sci. USA 106, 5813–5818 (2009).
Burant, C. F. et al. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 379, 1403–1411 (2012).
Assmann, A., Hinault, C. & Kulkarni, R. N. Growth factor control of pancreatic islet regeneration and function. Pediatr. Diabetes 10, 14–32 (2009).
Vasavada, R. C. et al. Protein kinase C-ζ activation markedly enhances β-cell proliferation: an essential role in growth factor mediated β-cell mitogenesis. Diabetes 56, 2732–2743 (2007).
Wiederkehr, A. & Wollheim, C. B. Mitochondrial signals drive insulin secretion in the pancreatic β-cell. Mol. Cell. Endocrinol. 353, 128–137 (2012).
Wang, C. et al. Deficiency of APPL1 in mice impairs glucose-stimulated insulin secretion through inhibition of pancreatic beta cell mitochondrial function. Diabetologia 56, 1999–2009 (2013).
Sivitz, W. I. & Yorek, M. A. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. Antioxid. Redox Signal. 12, 537–577 (2010).
Li, N., Stojanovski, S. & Maechler, P. Mitochondrial hormesis in pancreatic β cells: does uncoupling protein 2 play a role? Oxid. Med. Cell. Longev. 2012, 740849 (2012).
Aquilano, K., Baldelli, S., Pagliei, B. & Ciriolo, M. R. Extranuclear localization of SIRT1 and PGC-1α: an insight into possible roles in diseases associated with mitochondrial dysfunction. Curr. Mol. Med. 13, 140–154 (2013).
Matschinsky, F. M. et al. Glucokinase activators for diabetes therapy: May 2010 status report. Diabetes Care 34, S236–S243 (2011).
Engel, S. S. Glycemic and lipid effects of the short-acting glucagon receptor antagonist MK-3577 in patients with type 2 diabetes. Diabetes Abstr. 61, A266 (2012).
Gumbiner, B. Pronounced glucose (G) reduction in poorly controlled T2DM with MB07803, a novel fructose-1, 6-biphosphatase inhibitor (FBPasel) with reduced potential for acid-base disturbance versus the 1st generation FBPasel CS-917. Diabetes Abstr. 58, LB4 (2009).
Kumashiro, N. et al. Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes 62, 2183–2194 (2013).
Stark, R. et al. A role for mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) in the regulation of hepatic gluconeogenesis. J. Biol. Chem. 289, 7257–7263 (2014).
Harlan, D. M., Kenyon, N. S., Korsgren, O. & Roep, B. O. Current advances and travails in islet transplantation. Diabetes 58, 2175–2184 (2009).
Motté, E. et al. Composition and function of macroencapsulated human embryonic stem cell-derived implants: comparison with clinical human islet cell grafts. Am. J. Physiol. Endocrinol. Metab. 307, E838–E846 (2014).
Pagliuca, F. W. et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014).
Blum, B. et al. Reversal of β cell de-differentiation by a small molecule inhibitor of the TGFβ pathway. eLife 3, e02809 (2014).
Pickup, J. C. Banting Memorial Lecture 2014* Technology and diabetes care: appropriate and personalized. Diabet. Med. 32, 3–13 (2015).
Peyser, T., Dassau, E., Breton, M. & Skyler, J. S. The artificial pancreas: current status and future prospects in the management of diabetes. Ann. NY Acad. Sci. 1311, 102–123 (2014). This article presents an up-to-to-date status report on progress with the artificial pancreas (closed-loop system).
Klonoff, D. C. Afrezza inhaled insulin: the fastest-acting FDA-approved insulin on the market has favorable properties. J. Diabetes Sci. Technol. 8, 1071–1073 (2014).
Eldor, R., Arbit, E., Corcos, A. & Kidron, M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLoS ONE 8, e59524 (2013).
DeFronzo, R. A. Dissociation between metformin plasma exposure and its glucose-lowering effect: a novel gut-mediated mechanism of action. Diabetes 62, a281 (2013).
DePaoli, A. M., Higgins, L. S., Henry, R. R., Mantzoros, C. & Dunn, F. L. Can a selective PPARγ modulator improve glycemic control in patients with type 2 diabetes with fewer side effects compared with pioglitazone? Diabetes Care 37, 1918–1923 (2014).
Colca, J. R., Tanis, S. P., McDonald, W. G. & Kletzien, R. F. Insulin sensitizers in 2013: new insights for the development of novel therapeutic agents to treat metabolic diseases. Expert Opin. Investig. Drugs 23, 1–7 (2014).
Suh, J. M. et al. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature 513, 436–439 (2014).
Gaich, G. et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340 (2013).
Jeoung, N. H. & Harris, R. A. Role of pyruvate dehydrogenase kinase 4 in regulation of blood glucose levels. Korean Diabetes J. 34, 274–283 (2010).
Povel, C. M. et al. Metabolic syndrome model definitions predicting type 2 diabetes and cardiovascular disease. Diabetes Care 36, 362–368 (2013).
Pacini, G., Mari, A., Fouqueray, P., Bolze, S. & Roden, M. Imeglimin increases glucose-dependent insulin secretion and improves β-cell function in patients with type 2 diabetes. Diabetes Obes. Metab. 17, 541–545 (2015).
Birch, A. M., Buckett, L. K. & Turnbull, A. V. DGAT1 inhibitors as anti-obesity and anti-diabetic agents. Curr. Opin. Drug Discov. Devel. 13, 489–496 (2010).
Liu, L. et al. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat-induced insulin resistance. J. Clin. Invest. 117, 1679–1689 (2007).
Harrima, G., Greenwood, J. & Bhar, S. Acetyl-CoA carboxylase inhibition by NDI-630 inhibits fatty acid synthesis stimulates fatty acid oxidative, reduces body weight, improvise insulin sensitivity, and modulates dyslipidemia in rats. Diabetes Abstr. 62, A161 (2013).
Tao, H., Zhang, Y., Zeng, X., Shulman, G. I. & Jin, S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 20, 1263–1269 (2014).
Perry, R. J. et al. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metab. 18, 740–748 (2013).
Garvey, W. T. et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am. J. Clin. Nutr. 95, 297–308 (2012).
Carlsson, L. M. et al. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N. Engl. J. Med. 367, 695–704 (2012). The effectiveness and safety of bariatric surgery in the treatment of obesity and T2DM is reviewed in this longest ongoing study on surgical intervention.
Neuschwander-Tetri, B. A. et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 385, 956–965 (2014).
Out, C., Groen, A. K. & Brufau, G. Bile acid sequestrants: more than simple resins. Curr. Opin. Lipidol. 23, 43–55 (2012).
Cellitti, S. A novel GLP-1-FGF21 fusion protein for the treatment of diabetes and obesity. Keystone Symp. Obes. (2014).
Thareja, S., Aggarwal, S., Bhardwaj, T. R. & Kumar, M. Protein tyrosine phosphatase 1B inhibitors: a molecular level legitimate approach for the management of diabetes mellitus. Med. Res. Rev. 32, 459–517 (2012).
Chakraborty, C., Doss, C. G., Bandyopadhyay, S. & Agoramoorthy, G. Influence of miRNA in insulin signaling pathway and insulin resistance: micro-molecules with a major role in type-2 diabetes. Wiley Interdiscip. Rev. RNA 5, 697–712 (2014).
Tilg, H. & Moschen, A. R. Microbiota and diabetes: an evolving relationship. Gut 63, 1513–1521 (2014).
Patel, S. R., Hakim, D., Mason, J. & Hakim, N. The duodenal–jejunal bypass sleeve (EndoBarrier Gastrointestinal Liner) for weight loss and treatment of type 2 diabetes. Surg. Obes. Relat. Dis. 9, 482–484 (2013).
Bhatt, M. P., Lim, Y.-C. & Ha, K.-S. C-peptide replacement therapy as an emerging strategy for preventing diabetic vasculopathy. Cardiovasc. Res. 104, 234–244 (2014).
Bhat, M., Pouliot, M., Couture, R. & Vaucher, E. The kallikrein–kinin system in diabetic retinopathy. Prog. Drug Res. 69, 111–143 (2014).
Hajhosseiny, R. et al. Have we reached the limits for the treatment of diabetic nephropathy? Expert Opin. Investig. Drugs 23, 511–522 (2014).
Williams, M. E. et al. Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am. J. Nephrol. 27, 605–614 (2007).
De Zeeuw, D. et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 25, 1083–1093 (2014).
Boussageon, R. et al. Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. BMJ 343, d4169 (2011).
Colditz, G. A., Willett, W. C., Rotnitzky, A. & Manson, J. E. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann. Intern. Med. 122, 481–486 (1995).
Chan, J. M., Rimm, E. B., Colditz, G. A., Stampfer, M. J. & Willett, W. C. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17, 961–969 (1994).
Acknowledgements
The authors acknowledge grants from: the South Texas Veterans Healthcare System to R.A.D.; the National Institutes of Health (grants R01DK24092 to R.A.D.; DK58845 and P30 DK46200 to F.B.H.; R01 DK-040936, R01 DK-049230, R24 DK-085836, UL1 RR-045935, R01 DK-082659 and R24 DK085610 to G.I.S.; P30 DK036836 to C.R.K. Novo Nordisk Foundation for Basic Metabolic Research and the University of Copenhagen to G.I.S. and C.R.K.; DVA-Merit Review grant and VA San Diego Healthcare System to R.H.; National Institute for Diabetes and Digestive and Kidney Disease (grant P30DK092926) to W.H.; the Swedish Research Council (grants 2010–3490 and 2008–6589) and European Council (grants GA269045) to L.G.; Italian Ministry of University & Research (MIUR 2010329EKE) to E.F.; the Patient-Centered Outcomes Research Institute (PCORI) Program Award (CE1304-6756) to D.C.S. and M.A.T.; NovoNordisk Foundation to the NNF Center for Basic Metabolic Research to J.H. W.H. acknowledges the Michigan Center for Diabetes Translational Research and I.R. thanks R. Sprung for editorial assistance.
Author information
Authors and Affiliations
Contributions
Introduction (R.R.H.); Epidemiology (F.B.H.); Mechanisms/pathophysiology (L.C.G., C.R.K., E.F., G.I.S. and R.A.D.); Diagnosis, screening and prevention (W.H.H.); Management (R.A.D.); Quality of life (D.C.S. and M.A.T.); Outlook (I.R., J.J.H. and R.W.); overview of Primer (R.A.D.).
Corresponding author
Ethics declarations
Competing interests
The authors declare the following potential COI: (1) R.A.D.: Research Grant Support - AstraZeneca, Bristol Myers Squibb, Janssen; Speaker's Bureau - AstraZeneca, Novo Nordisk, Advisory Board/Consultant - AstraZeneca, Janssen, Novo Nordisk, Boehringer Ingelheim, Lexicon, Intarcia; (2) E.F.: Research Grant Support - Boehringer Ingelheim, Eli Lilly; Consultant/Speaker Bureau-Boehringer Ingelheim, Eli Lilly, Sanofi, Novo Nordisk, Janssen, AstraZeneca, Takeda, Medtronic, Intarcia; (3) C.R.K. serves as a consultant for Medimmune, Merck, Five Prime Therapeutics, CohBar, Antriabio, and Catabasis; (4) L.G. has no conflict of interest; (5) R.H. has received grant support from Hitachi, Janssen, Eli Lilly, Sanofi-Aventis and Viacyte and is a consultant/advisory board member for Alere, Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Clin Met, Eisai, Elcelyx, Gilead, Intarcia, Isis, Janssen, Merck, Novo Nordisk, Sanofi-Aventis, and Vivus; (6) W.H.H. has no conflict of interest; (7) J.J.H. has received grant support from Novartis and Merck and is a consultant/advisory board member for Glaxo, Smith, Kline, Novo Nordisk, and Zealand Pharmaceuticals; (8) M.A.T. has no conflict of interest; (9) R.W. serves as a consultant for Medtronics and Kamada and is on the speaker's bureau for Medtronics and Novo Nordisk; (10) F.H. has received research support from California Walnut Commission and Metegenics; (11) G.I.S. serves on scientific advisory boards for Merck and Novartis and he has received research grant support from Gilead Pharmaceuticals; (12) D.C.S. has no conflict of interest; (13) I.R. – Advisory Board: Novo Nordisk, Astra Zeneca/BMS, MSD, Eli Lilly, Sanofi, Medscape Cardiology; Consultant: Astra Zeneca/BMS, Insuline; Speaker's Bureau: Eli Lilly, Novo Nordisk, Astra Zeneca/BMS, J&J, Sanofi, MSD, Novartis, Teva; Shareholder: Insuline, Labstyle.
Rights and permissions
About this article
Cite this article
DeFronzo, R., Ferrannini, E., Groop, L. et al. Type 2 diabetes mellitus. Nat Rev Dis Primers 1, 15019 (2015). https://doi.org/10.1038/nrdp.2015.19
Published:
DOI: https://doi.org/10.1038/nrdp.2015.19
This article is cited by
-
The association between triglyceride-glucose index and its combination with obesity indicators and cardiovascular disease: NHANES 2003–2018
Cardiovascular Diabetology (2024)
-
Deep learning enables the quantification of browning capacity of human adipose samples
Journal of Big Data (2024)
-
Comparative impact of Roux-en-Y gastric bypass, sleeve gastrectomy or diet alone on beta-cell function in insulin-treated type 2 diabetes patients
Scientific Reports (2024)
-
Differences in gut microbiota between Dutch and South-Asian Surinamese: potential implications for type 2 diabetes mellitus
Scientific Reports (2024)
-
Biosynthesized copper oxide nanoparticles by Psidium guajava plants with antibacterial, antidiabetic, antioxidant, and photocatalytic capacity
Biomass Conversion and Biorefinery (2024)