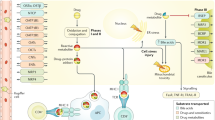
Abstract
Methotrexate is a key component of the treatment of inflammatory rheumatic diseases and the mainstay of therapy in rheumatoid arthritis. Hepatotoxicity has long been a concern for prescribers envisaging long-term treatment with methotrexate for their patients. However, the putative liver toxicity of methotrexate should be evaluated in the context of advances in our knowledge of the pathogenesis and natural history of liver disease, especially non-alcoholic fatty liver disease (NAFLD). Notably, patients with NAFLD are at increased risk for methotrexate hepatotoxicity, and methotrexate can worsen the course of NAFLD. Understanding the mechanisms of acute hepatotoxicity can facilitate the interpretation of elevated concentrations of liver enzymes in this context. Liver fibrosis and the mechanisms of fibrogenesis also need to be considered in relation to chronic exposure to methotrexate. A number of non-invasive tests for liver fibrosis are available for use in patients with rheumatic disease, in addition to liver biopsy, which can be appropriate for particular individuals. On the basis of the available evidence, practical suggestions for pretreatment screening and long-term monitoring of methotrexate therapy can be made for patients who have (or are at risk for) chronic liver disease.
Key points
-
Methotrexate is a key component in the treatment of inflammatory rheumatic diseases and the mainstay of therapy in rheumatoid arthritis.
-
In light of current evidence, it seems unlikely that methotrexate alone is capable of inducing chronic liver disease; the risk of methotrexate-induced liver injury is primarily acute in nature.
-
The cumulative dose of methotrexate has no predictive value for the occurrence of fibrosis.
-
In non-alcoholic fatty liver disease, several pathophysiological arguments suggest (in the absence of proof from clinical trials) that long-term methotrexate therapy worsens liver damage and the progression of liver disease.
-
Pretreatment screening is advisable to check for the possible presence of liver disease in patients being considered for methotrexate treatment.
-
Monthly monitoring is advocated at the beginning of methotrexate treatment, followed by 3-monthly monitoring comprising complete blood counts, liver function tests and calculation of the Fib-4 fibrosis score.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Smolen, J. S. et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann. Rheum. Dis. 75, 3–15 (2016).
Visser, K. & van der Heijde, D. Optimal dosage and route of administration of methotrexate in rheumatoid arthritis: a systematic review of the literature. Ann. Rheum. Dis. 68, 1094–1099 (2009).
Friedman, B. & Cronstein, B. Methotrexate mechanism in treatment of rheumatoid arthritis. Joint Bone Spine 86, 301–307 (2019).
Sherbini, A. A., Sharma, S. D., Gwinnutt, J. M., Hyrich, K. L. & Verstappen, S. M. M. Prevalence and predictors of adverse events with methotrexate mono- and combination-therapy for rheumatoid arthritis: a systematic review. Rheumatology 60, 4001–4017 (2021).
Juge, P. A. et al. Methotrexate and rheumatoid arthritis associated interstitial lung disease. Eur. Respir. J. 57, 2000337 (2021).
Elsawy, H. et al. Naringin alleviates methotrexate-induced liver injury in male albino rats and enhances its antitumor efficacy in HepG2 cells. Biosci. Rep. 40, BSR20193686 (2020).
Ezhilarasan, D. Hepatotoxic potentials of methotrexate: Understanding the possible toxicological molecular mechanisms. Toxicology 458, 152840 (2021).
Cure, E. et al. Protective effect of infliximab on methotrexate-induced liver injury in rats: unexpected drug interaction. J. Cancer Res. Ther. 11, 164–169 (2015).
van Ede, A. E. et al. Homocysteine and folate status in methotrexate-treated patients with rheumatoid arthritis. Rheumatology 41, 658–665 (2002).
Goudarzi, M., Kalantar, M., Sadeghi, E., Karamallah, M. H. & Kalantar, H. Protective effects of apigenin on altered lipid peroxidation, inflammation, and antioxidant factors in methotrexate-induced hepatotoxicity. Naunyn Schmiedebergs Arch. Pharmacol. 394, 523–531 (2021).
Ali, N. et al. Protective effect of Chlorogenic acid against methotrexate induced oxidative stress, inflammation and apoptosis in rat liver: an experimental approach. Chem. Biol. Interact. 272, 80–91 (2017).
Abo-Haded, H. M., Elkablawy, M. A., Al-Johani, Z., Al-Ahmadi, O. & El-Agamy, D. S. Hepatoprotective effect of sitagliptin against methotrexate induced liver toxicity. PLoS One 12, e0174295 (2017).
Kobayashi, K., Terada, C. & Tsukamoto, I. Methotrexate-induced apoptosis in hepatocytes after partial hepatectomy. Eur. J. Pharmacol. 438, 19–24 (2002).
Ashok, I. & Sheeladevi, R. Oxidant stress evoked damage in rat hepatocyte leading to triggered nitric oxide synthase (NOS) levels on long term consumption of aspartame. J. Food Drug Anal. 23, 679–691 (2015).
Al Kury, L. T. et al. Ginkgo biloba extract protects against methotrexate-induced hepatotoxicity: a computational and pharmacological approach. Molecules 25, 2540 (2020).
Chauhan, P. et al. Protective effects of Glycyrrhiza glabra supplementation against methotrexate-induced hepato-renal damage in rats: an experimental approach. J. Ethnopharmacol. 263, 113209 (2020).
Yao, P., He, X., Zhang, R., Tong, R. & Xiao, H. The influence of MTHFR genetic polymorphisms on adverse reactions after methotrexate in patients with hematological malignancies: a meta-analysis. Hematology 24, 10–19 (2019).
Conway, R., Low, C., Coughlan, R. J., O’Donnell, M. J. & Carey, J. J. Risk of liver injury among methotrexate users: a meta-analysis of randomised controlled trials. Semin. Arthritis Rheum. 45, 156–162 (2015).
Khan, N. et al. Incidence of liver toxicity in inflammatory bowel disease patients treated with methotrexate: a meta-analysis of clinical trials. Inflamm. Bowel Dis. 18, 359–367 (2012).
Solomon, D. H. et al. Adverse effects of low-dose methotrexate: a randomized trial. Ann. Intern. Med. 172, 369–380 (2020).
Sajith, M., Pawar, A., Bafna, V. & Bartakke, S. High-dose methotrexate-induced fulminant hepatic failure and pancytopenia in an acute lymphoblastic leukaemia paediatric patient. Eur. J. Hosp. Pharm. 27, 178–180 (2020).
National Institute of Diabetes and Digestive and Kidney Diseases. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury https://www.ncbi.nlm.nih.gov/books/NBK547852/ (2012).
Friedman, S. L. Mechanisms of hepatic fibrogenesis. Gastroenterology 134, 1655–1669 (2008).
Henderson, N. C. & Forbes, S. J. Hepatic fibrogenesis: from within and outwith. Toxicology 254, 130–135 (2008).
Sherif, I. O. & Al-Shaalan, N. H. Hepatoprotective effect of Ginkgo biloba extract against methotrexate-induced hepatotoxicity via targeting STAT3/miRNA-21 axis. Drug Chem. Toxicol. 45, 1723–1731 (2022).
Zhao, J., Qi, Y. F. & Yu, Y. R. STAT3: a key regulator in liver fibrosis. Ann. Hepatol. 21, 100224 (2021).
Ezhilarasan, D. MicroRNA interplay between hepatic stellate cell quiescence and activation. Eur. J. Pharmacol. 885, 173507 (2020).
Taft, L. I. Methotrexate induced hepatitis in childhood leukemia. Isr. J. Med. Sci. 1, 823–827 (1965).
Coe, R. O. & Bull, F. E. Cirrhosis associated with methotrexate treatment of psoriasis. JAMA 206, 1515–1520 (1968).
Themido, R., Loureiro, M., Pecegueiro, M., Brandao, M. & Campos, M. C. Methotrexate hepatotoxicity in psoriatic patients submitted to long-term therapy. Acta Derm. Venereol. 72, 361–364 (1992).
Zachariae, H., Kragballe, K. & Sogaard, H. Methotrexate induced liver cirrhosis. Studies including serial liver biopsies during continued treatment. Br. J. Dermatol. 102, 407–412 (1980).
Cheng, H. S. & Rademaker, M. Monitoring methotrexate-induced liver fibrosis in patients with psoriasis: utility of transient elastography. Psoriasis 8, 21–29 (2018).
Roenigk, H. H. Jr., Auerbach, R., Maibach, H. I. & Weinstein, G. D. Methotrexate in psoriasis: revised guidelines. J. Am. Acad. Dermatol. 19, 145–156 (1988).
Whiting-O’Keefe, Q. E., Fye, K. H. & Sack, K. D. Methotrexate and histologic hepatic abnormalities: a meta-analysis. Am. J. Med. 90, 711–716 (1991).
Kremer, J. M. et al. Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. American College of Rheumatology. Arthritis Rheum. 37, 316–328 (1994).
Rubbia-Brandt, L. et al. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J. Hepatol. 33, 106–115 (2000).
Lok, A. S. & Gunaratnam, N. T. Diagnosis of hepatitis C. Hepatology 26, 48S–56S (1997).
Sanyal, A. J. Past, present and future perspectives in nonalcoholic fatty liver disease. Nat. Rev. Gastroenterol. Hepatol. 16, 377–386 (2019).
Langman, G., Hall, P. M. & Todd, G. Role of non-alcoholic steatohepatitis in methotrexate-induced liver injury. J. Gastroenterol. Hepatol. 16, 1395–1401 (2001).
Klujszo, E. H., Parcheta, P., Witkowska, A. B. & Krecisz, B. Non-alcoholic fatty liver disease in patients with psoriasis: therapeutic implications. Postepy Dermatol. Alergol. 37, 468–474 (2020).
Roberts, K. K. et al. The prevalence of NAFLD and NASH among patients with psoriasis in a tertiary care dermatology and rheumatology clinic. Aliment. Pharmacol. Ther. 41, 293–300 (2015).
Miele, L. et al. Prevalence, characteristics and severity of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis. J. Hepatol. 51, 778–786 (2009).
Mori, S. et al. Non-alcoholic steatohepatitis-like pattern in liver biopsy of rheumatoid arthritis patients with persistent transaminitis during low-dose methotrexate treatment. PLoS One 13, e0203084 (2018).
Gelfand, J. M. et al. Risk of liver disease in patients with psoriasis, psoriatic arthritis, and rheumatoid arthritis receiving methotrexate: a population-based study. J. Am. Acad. Dermatol. 84, 1636–1643 (2021).
Roenigk, H. H. Jr., Bergfeld, W. F., St Jacques, R., Owens, F. J. & Hawk, W. A. Hepatotoxicity of methotrexate in the treatment of psoriasis. Arch. Dermatol. 103, 250–261 (1971).
Te, H. S. et al. Hepatic effects of long-term methotrexate use in the treatment of inflammatory bowel disease. Am. J. Gastroenterol. 95, 3150–3156 (2000).
Maybury, C. M., Samarasekera, E., Douiri, A., Barker, J. N. & Smith, C. H. Diagnostic accuracy of noninvasive markers of liver fibrosis in patients with psoriasis taking methotrexate: a systematic review and meta-analysis. Br. J. Dermatol. 170, 1237–1247 (2014).
de Ledinghen, V. et al. Diagnostic and predictive factors of significant liver fibrosis and minimal lesions in patients with persistent unexplained elevated transaminases. A prospective multicenter study. J. Hepatol. 45, 592–599 (2006).
Verma, S., Jensen, D., Hart, J. & Mohanty, S. R. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 33, 1398–1405 (2013).
Younossi, Z. M. et al. Clinical assessment for high-risk patients with non-alcoholic fatty liver disease in primary care and diabetology practices. Aliment. Pharmacol. Ther. 52, 513–526 (2020).
Hagstrom, H., Talback, M., Andreasson, A., Walldius, G. & Hammar, N. Repeated FIB-4 measurements can help identify individuals at risk of severe liver disease. J. Hepatol. 73, 1023–1029 (2020).
Castera, L. et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128, 343–350 (2005).
Castera, L. Non-invasive tests for liver fibrosis in NAFLD: creating pathways between primary healthcare and liver clinics. Liver Int. 40, 77–81 (2020).
Crossan, C. et al. Referral pathways for patients with NAFLD based on non-invasive fibrosis tests: diagnostic accuracy and cost analysis. Liver Int. 39, 2052–2060 (2019).
Boursier, J. et al. Non-invasive diagnosis and follow-up of non-alcoholic fatty liver disease. Clin. Res. Hepatol. Gastroenterol. 46, 101769 (2021).
Labadie, J. G. & Jain, M. Noninvasive tests to monitor methotrexate-induced liver injury. Clin. Liver Dis. 13, 67–71 (2019).
Lynch, M. et al. The use of transient elastography and FibroTest for monitoring hepatotoxicity in patients receiving methotrexate for psoriasis. JAMA Dermatol. 150, 856–862 (2014).
Cervoni, J. P. et al. A pragmatic non-invasive assessment of liver fibrosis in patients with psoriasis, rheumatoid arthritis or Crohn’s disease receiving methotrexate therapy. Clin. Res. Hepatol. Gastroenterol. 44S, 100003 (2020).
Laharie, D. et al. Assessment of liver fibrosis with transient elastography and FibroTest in patients treated with methotrexate for chronic inflammatory diseases: a case-control study. J. Hepatol. 53, 1035–1040 (2010).
Kim, T. Y. et al. Assessment of substantial liver fibrosis by real-time shear wave elastography in methotrexate-treated patients with rheumatoid arthritis. J. Ultrasound Med. 34, 1621–1630 (2015).
Aithal, G. P. et al. Monitoring methotrexate-induced hepatic fibrosis in patients with psoriasis: are serial liver biopsies justified? Aliment. Pharmacol. Ther. 19, 391–399 (2004).
Laharie, D. et al. Diagnosis of liver fibrosis by transient elastography (FibroScan) and non-invasive methods in Crohn’s disease patients treated with methotrexate. Aliment. Pharmacol. Ther. 23, 1621–1628 (2006).
Azzam, A., Jiyad, Z. & O’Beirne, J. Is methotrexate hepatotoxicity associated with cumulative dose? A systematic review and meta-analysis. Australas. J. Dermatol. 62, 130–140 (2021).
Cheema, H. I., Haselow, D. & Dranoff, J. A. Review of existing evidence demonstrates that methotrexate does not cause liver fibrosis. J. Investig. Med. 70, 1452–1460 (2022).
Gisondi, P., Fostini, A. C., Fossa, I., Girolomoni, G. & Targher, G. Psoriasis and the metabolic syndrome. Clin. Dermatol. 36, 21–28 (2018).
Gisondi, P. et al. Prevalence of metabolic syndrome in patients with psoriasis: a hospital-based case-control study. Br. J. Dermatol. 157, 68–73 (2007).
Loganathan, A., Kamalaraj, N., El-Haddad, C. & Pile, K. Systematic review and meta-analysis on prevalence of metabolic syndrome in psoriatic arthritis, rheumatoid arthritis and psoriasis. Int. J. Rheum. Dis. 24, 1112–1120 (2021).
Verhoeven, F., Prati, C., Demougeot, C. & Wendling, D. Cardiovascular risk in psoriatic arthritis, a narrative review. Joint Bone Spine 87, 413–418 (2020).
da Cunha, V. R. et al. Metabolic syndrome prevalence is increased in rheumatoid arthritis patients and is associated with disease activity. Scand. J. Rheumatol. 41, 186–191 (2012).
Zonana-Nacach, A., Santana-Sahagun, E., Jimenez-Balderas, F. J. & Camargo-Coronel, A. Prevalence and factors associated with metabolic syndrome in patients with rheumatoid arthritis and systemic lupus erythematosus. J. Clin. Rheumatol. 14, 74–77 (2008).
Gremese, E. & Ferraccioli, G. The metabolic syndrome: the crossroads between rheumatoid arthritis and cardiovascular risk. Autoimmun. Rev. 10, 582–589 (2011).
Meune, C., Touze, E., Trinquart, L. & Allanore, Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology 48, 1309–1313 (2009).
European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) & European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 64, 1388–1402 (2016).
Dulai, P. S. et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 65, 1557–1565 (2017).
Armandi, A. & Bugianesi, E. Natural history of NASH. Liver Int. 41, 78–82 (2021).
Powell, E. E., Wong, V. W. & Rinella, M. Non-alcoholic fatty liver disease. Lancet 397, 2212–2224 (2021).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64, 73–84 (2016).
Estes, C., Razavi, H., Loomba, R., Younossi, Z. & Sanyal, A. J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 67, 123–133 (2018).
Parlati, L., Regnier, M., Guillou, H. & Postic, C. New targets for NAFLD. JHEP Rep. 3, 100346 (2021).
Parthasarathy, G., Revelo, X. & Malhi, H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol. Commun. 4, 478–492 (2020).
Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013).
Krawczyk, M., Liebe, R.&Lammert, F. Toward genetic prediction of nonalcoholic fatty liver disease trajectories: PNPLA3 and beyond. Gastroenterology 158, 1865–1880.e1 (2020).
Massart, J., Begriche, K., Moreau, C. & Fromenty, B. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J. Clin. Transl. Res. 3, 212–232 (2017).
Kang, S. W. et al. AMPK activation prevents and reverses drug-induced mitochondrial and hepatocyte injury by promoting mitochondrial fusion and function. PLoS One 11, e0165638 (2016).
Mansouri, A., Gattolliat, C. H. & Asselah, T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology 155, 629–647 (2018).
Jaeschke, H., McGill, M. R. & Ramachandran, A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 44, 88–106 (2012).
Pessayre, D. et al. Central role of mitochondria in drug-induced liver injury. Drug Metab. Rev. 44, 34–87 (2012).
Ye, H., Nelson, L. J., Gomez Del Moral, M., Martinez-Naves, E. & Cubero, F. J. Dissecting the molecular pathophysiology of drug-induced liver injury. World J. Gastroenterol. 24, 1373–1385 (2018).
Pessayre, D. & Fromenty, B. NASH: a mitochondrial disease. J. Hepatol. 42, 928–940 (2005).
Chowdhry, S. et al. Loss of Nrf2 markedly exacerbates nonalcoholic steatohepatitis. Free. Radic. Biol. Med. 48, 357–371 (2010).
Solano-Urrusquieta, A. et al. NRF-2 and nonalcoholic fatty liver disease. Ann. Hepatol. 19, 458–465 (2020).
Wright, A. J., Dainty, J. R. & Finglas, P. M. Folic acid metabolism in human subjects revisited: potential implications for proposed mandatory folic acid fortification in the UK. Br. J. Nutr. 98, 667–675 (2007).
Stover, P. J. & Field, M. S. Trafficking of intracellular folates. Adv. Nutr. 2, 325–331 (2011).
Au-Yeung, K. K., Yip, J. C., Siow, Y. L. & O, K. Folic acid inhibits homocysteine-induced superoxide anion production and nuclear factor kappa B activation in macrophages. Can. J. Physiol. Pharmacol. 84, 141–147 (2006).
Sid, V., Siow, Y. L. & O, K. Role of folate in nonalcoholic fatty liver disease. Can. J. Physiol. Pharmacol. 95, 1141–1148 (2017).
Zhu, L. et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 57, 601–609 (2013).
Rossi, M., Amaretti, A. & Raimondi, S. Folate production by probiotic bacteria. Nutrients 3, 118–134 (2011).
Sid, V., Siow, Y. L., Shang, Y., Woo, C. W. & O, K. High-fat diet consumption reduces hepatic folate transporter expression via nuclear respiratory factor-1. J. Mol. Med. 96, 1203–1213 (2018).
Koplay, M., Gulcan, E. & Ozkan, F. Association between serum vitamin B12 levels and the degree of steatosis in patients with nonalcoholic fatty liver disease. J. Investig. Med. 59, 1137–1140 (2011).
da Silva, R. P., Kelly, K. B., Al Rajabi, A. & Jacobs, R. L. Novel insights on interactions between folate and lipid metabolism. Biofactors 40, 277–283 (2014).
Vahedi, H., Bavafaetousi, N., Zolfaghari, P., Yarmohammadi, M. & Bagher Sohrabi, M. Association between serum folate levels and fatty liver disease. Clin. Nutr. Exp. 29, 30–35 (2020).
Christensen, K. E. et al. Steatosis in mice is associated with gender, folate intake, and expression of genes of one-carbon metabolism. J. Nutr. 140, 1736–1741 (2010).
Champier, J., Claustrat, F., Nazaret, N., Fevre Montange, M. & Claustrat, B. Folate depletion changes gene expression of fatty acid metabolism, DNA synthesis, and circadian cycle in male mice. Nutr. Res. 32, 124–132 (2012).
Kim, Y. I. et al. Severe folate deficiency causes secondary depletion of choline and phosphocholine in rat liver. J. Nutr. 124, 2197–2203 (1994).
Bird, J. K. et al. Obesity is associated with increased red blood cell folate despite lower dietary intakes and serum concentrations. J. Nutr. 145, 79–86 (2015).
Mojtabai, R. Body mass index and serum folate in childbearing age women. Eur. J. Epidemiol. 19, 1029–1036 (2004).
Hirsch, S. et al. Serum folate and homocysteine levels in obese females with non-alcoholic fatty liver. Nutrition 21, 137–141 (2005).
Mahamid, M. et al. Folate and B12 levels correlate with histological severity in NASH patients. Nutrients 10, 440 (2018).
Tripathi, M. et al. Vitamin B12 and folate decrease inflammation and fibrosis in NASH by preventing Syntaxin 17 homocysteinylation. J. Hepatol. 77, 1246–1255 (2022).
Sid, V. et al. Folic acid supplementation during high-fat diet feeding restores AMPK activation via an AMP-LKB1-dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R1215–R1225 (2015).
Sarna, L. K. et al. Folic acid supplementation attenuates high fat diet induced hepatic oxidative stress via regulation of NADPH oxidase. Can. J. Physiol. Pharmacol. 90, 155–165 (2012).
Sid, V. et al. Folic acid supplementation attenuates chronic hepatic inflammation in high-fat diet fed mice. Lipids 53, 709–716 (2018).
Chen, D.-Y. et al. Blood lipid profiles and peripheral blood mononuclear cell cholesterol metabolism gene expression in patients with and without methotrexate treatment. BMC Med. 9, 4 (2011).
Mori, S. et al. Incidence, predictive factors and severity of methotrexate-related liver injury in rheumatoid arthritis: a longitudinal cohort study. Rheumatol. Adv. Pract. 4, rkaa020 (2020).
Shea, B. Folic acid or folinic acid for reducing side effects of methotrexate for people with rheumatoid arthritis. J. Evid. Based Med. 6, 202–203 (2013).
Morgan, S. L. et al. The effect of folic acid supplementation on the toxicity of low-dose methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 33, 9–18 (1990).
Shiroky, J. B. et al. Low-dose methotrexate with leucovorin (folinic acid) in the management of rheumatoid arthritis. Results of a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 36, 795–803 (1993).
van Ede, A. E. et al. The C677T mutation in the methylenetetrahydrofolate reductase gene: a genetic risk factor for methotrexate-related elevation of liver enzymes in rheumatoid arthritis patients. Arthritis Rheum. 44, 2525–2530 (2001).
Weinblatt, M. E., Maier, A. L. & Coblyn, J. S. Low dose leucovorin does not interfere with the efficacy of methotrexate in rheumatoid arthritis: an 8 week randomized placebo controlled trial. J. Rheumatol. 20, 950–952 (1993).
Rosenberg, P. et al. Psoriasis patients with diabetes type 2 are at high risk of developing liver fibrosis during methotrexate treatment. J. Hepatol. 46, 1111–1118 (2007).
Dawwas, M. F. & Aithal, G. P. End-stage methotrexate-related liver disease is rare and associated with features of the metabolic syndrome. Aliment. Pharmacol. Ther. 40, 938–948 (2014).
Danan, G. Definitions and assessment criteria of acute drug-induced hepatitis. Conclusions of an International Consensus Meeting. Gastroenterol. Clin. Biol. 15, 845–848 (1991).
Treem, W. R. et al. Consensus guidelines: best practices for detection, assessment and management of suspected acute drug-induced liver injury during clinical trials in adults with chronic viral hepatitis and adults with cirrhosis secondary to hepatitis B, C and nonalcoholic steatohepatitis. Drug Saf. 44, 133–165 (2021).
Pavy, S. et al. Methotrexate therapy for rheumatoid arthritis: clinical practice guidelines based on published evidence and expert opinion. Joint Bone Spine 73, 388–395 (2006).
Halfon, P. et al. A prospective assessment of the inter-laboratory variability of biochemical markers of fibrosis (FibroTest) and activity (ActiTest) in patients with chronic liver disease. Comp. Hepatol. 1, 3 (2002).
Prati, D. et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann. Intern. Med. 137, 1–10 (2002).
Valenti, L. et al. Definition of healthy ranges for alanine aminotransferase levels: a 2021 update. Hepatol. Commun. 5, 1824–1832 (2021).
Warren, R. B. et al. British Association of Dermatologists’ guidelines for the safe and effective prescribing of methotrexate for skin disease 2016. Br. J. Dermatol. 175, 23–44 (2016).
Gyulai, R. et al. Current practice of methotrexate use for psoriasis: results of a worldwide survey among dermatologists. J. Eur. Acad. Dermatol. Venereol. 29, 224–231 (2015).
Kanwal, F. et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology 161, 1657–1669 (2021).
European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis — 2021 update. J. Hepatol. 75, 659–689 (2021).
Xiao, G. et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology 66, 1486–1501 (2017).
Mozes, F. E. et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis. Gut 71, 1006–1019 (2021).
Miyata, M. et al. Validation of the fibrosis-4 (FIB-4) index in the diagnosis of liver disease of rheumatoid arthritis patients treated with methotrexate. Mod. Rheumatol. 29, 936–942 (2019).
Kim, S. U. et al. Fibrosis-4 index at diagnosis can predict all-cause mortality in patients with rheumatoid arthritis: a retrospective monocentric study. Mod. Rheumatol. 30, 70–77 (2020).
Avouac, J. et al. Risk of liver fibrosis induced by methotrexate and other rheumatoid arthritis medications according to the Fibrosis-4 index. Clin. Exp. Rheumatol. 40, 150–157 (2021).
Olsson-White, D. A., Olynyk, J. K., Ayonrinde, O. T., Paramalingam, S. & Keen, H. I. Assessment of liver fibrosis markers in people with rheumatoid arthritis on methotrexate. Intern. Med. J. 52, 566–573 (2022).
Darabian, S. et al. Using fibroscan to assess for the development of liver fibrosis in patients with arthritis on methotrexate: a single-center experience. J. Rheumatol. 49, 558–565 (2022).
Frankowski, M. et al. Usefulness of noninvasive diagnostic procedures for assessment of methotrexate hepatotoxicity in patients with rheumatoid arthritis. Rheumatol. Int. 42, 631–638 (2022).
Bafna, P. et al. Prevalence of liver fibrosis by Fibroscan in patients on long-term methotrexate therapy for rheumatoid arthritis. Clin. Rheumatol. 40, 3605–3613 (2021).
Feuchtenberger, M., Kraus, L., Nigg, A., Schulze-Koops, H. & Schafer, A. Methotrexate does not increase the risk of liver fibrosis in patients with rheumatoid arthritis: assessment by ultrasound elastography (ARFI-MetRA study). Rheumatol. Int. 41, 1079–1087 (2021).
Khandpur, S. et al. Ultrasound liver elastography for the detection of liver fibrosis in patients with psoriasis and reactive arthritis on long-term methotrexate therapy: a cross-sectional study. Indian J. Dermatol. Venereol. Leprol. 86, 508–514 (2020).
Erre, G. L. et al. Methotrexate therapy is not associated with increased liver stiffness and significant liver fibrosis in rheumatoid arthritis patients: a cross-sectional controlled study with real-time two-dimensional shear wave elastography. Eur. J. Intern. Med. 69, 57–63 (2019).
Lertnawapan, R., Chonprasertsuk, S. & Siramolpiwat, S. Association between cumulative methotrexate dose, non-invasive scoring system and hepatic fibrosis detected by Fibroscan in rheumatoid arthritis patients receiving methotrexate. Int. J. Rheum. Dis. 22, 214–221 (2019).
Rouhi, A., Hazlewood, G., Shaheen, A. A., Swain, M. G. & Barber, C. E. H. Prevalence and risk factors for liver fibrosis detected by transient elastography or shear wave elastography in inflammatory arthritis: a systematic review. Clin. Exp. Rheumatol. 35, 1029–1036 (2017).
Barbero-Villares, A. et al. Evaluation of liver fibrosis by transient elastography in methotrexate treated patients. Med. Clin. 137, 637–639 (2011).
Park, S. H., Choe, J. Y. & Kim, S. K. Assessment of liver fibrosis by transient elastography in rheumatoid arthritis patients treated with methotrexate. Joint Bone Spine 77, 588–592 (2010).
Ledingham, J. et al. BSR and BHPR guideline for the prescription and monitoring of non-biologic disease-modifying anti-rheumatic drugs. Rheumatology 56, 865–868 (2017).
Duarte, A. C. et al. Portuguese recommendations for the use of methotrexate in rheumatic diseases - 2016 update. Acta Reumatol. Port. 42, 127–140 (2017).
Singh, J. A. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 68, 1–26 (2016).
Madsen, O. R. et al. Evidence-based recommendations for treatment with methotrexate in rheumatic disorders. Dan. Med. Bull. 57, A4190 (2010).
Pereira, I. A. et al. National recommendations based on scientific evidence and opinions of experts on the use of methotrexate in rheumatic disorders, especially in rheumatoid arthritis. Results of the 3E initiative from Brazil. Rev. Bras. Reumatol. 49, 346–361 (2009).
Menter, A. et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J. Am. Acad. Dermatol. 82, 1445–1486 (2020).
Raaby, L. et al. Methotrexate use and monitoring in patients with psoriasis: a consensus report based on a danish expert meeting. Acta Derm. Venereol. 97, 426–432 (2017).
Nast, A. et al. European S3–Guidelines on the systemic treatment of psoriasis vulgaris–Update 2015–Short version–EDF in cooperation with EADV and IPC. J. Eur. Acad. Dermatol. Venereol. 29, 2277–2294 (2015).
Zweegers, J. et al. Summary of the Dutch S3–guidelines on the treatment of psoriasis 2011. Dutch Society of Dermatology and Venereology. Dermatol. Online J. 20, doj_21769 (2014).
Carretero, G. et al. Guidelines on the use of methotrexate in psoriasis. Actas Dermosifiliogr. 101, 600–613 (2010).
Maybury, C. M. et al. Methotrexate and liver fibrosis in people with psoriasis: a systematic review of observational studies. Br. J. Dermatol. 171, 17–29 (2014).
Acknowledgements
The authors thank F. Ecarnot (EA3920, University of Franche-Comté and University Hospital Besançon, France) for editorial assistance.
Author information
Authors and Affiliations
Contributions
V.D.M., D.W.-V., F.V., F.A., J.A. and T.T. researched data for the article. V.D.M., D.W.-V., F.V., J.A., T.T. and D.W. wrote the article. All authors contributed substantially to the discussion of content and to the review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Rheumatology thanks Devaraj Ezhilarasan, Shunsuke Mori and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Di Martino, V., Verhoeven, D.W., Verhoeven, F. et al. Busting the myth of methotrexate chronic hepatotoxicity. Nat Rev Rheumatol 19, 96–110 (2023). https://doi.org/10.1038/s41584-022-00883-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41584-022-00883-4