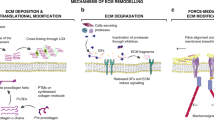
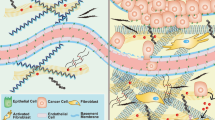
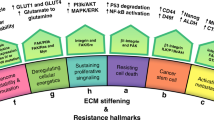
Abstract
The extracellular matrix is a fundamental, core component of all tissues and organs, and is essential for the existence of multicellular organisms. From the earliest stages of organism development until death, it regulates and fine-tunes every cellular process in the body. In cancer, the extracellular matrix is altered at the biochemical, biomechanical, architectural and topographical levels, and recent years have seen an exponential increase in the study and recognition of the importance of the matrix in solid tumours. Coupled with the advancement of new technologies to study various elements of the matrix and cell–matrix interactions, we are also beginning to see the deployment of matrix-centric, stromal targeting cancer therapies. This Review touches on many of the facets of matrix biology in solid cancers, including breast, pancreatic and lung cancer, with the aim of highlighting some of the emerging interactions of the matrix and influences that the matrix has on tumour onset, progression and metastatic dissemination, before summarizing the ongoing work in the field aimed at developing therapies to co-target the matrix in cancer and cancer metastasis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Yamada, K. M. et al. Extracellular matrix dynamics in cell migration, invasion and tissue morphogenesis. Int. J. Exp. Pathol. 100, 144–152 (2019).
Bissell, M. J., Hall, H. G. & Parry, G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 99, 31–68 (1982).
Cox, T. R. & Erler, J. T. Molecular pathways: connecting fibrosis and solid tumor metastasis. Clin. Cancer Res. 20, 3637–3643 (2014).
Tian, C. et al. Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc. Natl Acad. Sci. USA 116, 19609–19618 (2019). Study using proteomics on human-in-mouse tumour xenografts to dissect the contribution of tumour versus non-tumour cells to matrix deposition.
Naba, A. et al. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol. Cell Proteomics 11, M111.014647 (2012).
Kadler, K. E., Baldock, C., Bella, J. & Boot-Handford, R. P. Collagens at a glance. J. Cell Sci. 120, 1955–1958 (2007).
Ewald, C. Y. The matrisome during aging and longevity: a systems-level approach toward defining matreotypes promoting healthy aging. Gerontology https://doi.org/10.1159/000504295 (2019).
Kai, F., Drain, A. P. & Weaver, V. M. The extracellular matrix modulates the metastatic journey. Dev. Cell 49, 332–346 (2019).
Parsons, J. T., Horwitz, A. R. & Schwartz, M. A. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11, 633–643 (2010).
Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science 326, 1216–1219 (2009).
Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 3, a004978 (2011).
Fang, M., Yuan, J., Peng, C. & Li, Y. Collagen as a double-edged sword in tumor progression. Tumour Biol. 35, 2871–2882 (2014).
Sottile, J. & Hocking, D. C. Fibronectin polymerization regulates the composition and stability of extracellular matrix fibrils and cell-matrix adhesions. Mol. Biol. Cell 13, 3546–3559 (2002).
Piersma, B., Hayward, M. K. & Weaver, V. M. Fibrosis and cancer: a strained relationship. Biochim. Biophys. Acta Rev. Cancer 1873, 188356 (2020).
Provenzano, P. P. et al. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 4, 38 (2006). Early study showing how precise organization of collagen fibres at the tumour–stroma boundary plays a critical role in local invasion.
Amatangelo, M. D., Bassi, D. E., Klein-Szanto, A. J. P. & Cukierman, E. Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. Am. J. Pathol. 167, 475–488 (2005).
Amenta, P. S. et al. Type XV collagen in human colonic adenocarcinomas has a different distribution than other basement membrane zone proteins. Hum. Pathol. 31, 359–366 (2000).
Amenta, P. S. et al. Loss of types XV and XIX collagen precedes basement membrane invasion in ductal carcinoma of the female breast. J. Pathol. 199, 298–308 (2003).
Chang, J. & Chaudhuri, O. Beyond proteases: basement membrane mechanics and cancer invasion. J. Cell Biol. 218, 2456–2469 (2019).
Iozzo, R. V. & Schaefer, L. Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 42, 11–55 (2015).
Vitale, D. et al. Proteoglycans and glycosaminoglycans as regulators of cancer stem cell function and therapeutic resistance. FEBS J. 286, 2870–2882 (2019).
Iozzo, R. V. Matrix proteoglycans: from molecular design to cellular function. Annu. Rev. Biochem. 67, 609–652 (1998).
Bohaumilitzky, L. et al. A trickster in disguise: hyaluronan’s ambivalent roles in the matrix. Front. Oncol. 7, 242 (2017).
Price, Z. K., Lokman, N. A. & Ricciardelli, C. Differing roles of hyaluronan molecular weight on cancer cell behavior and chemotherapy resistance. Cancers 10, 482 (2018).
Caon, I. et al. Revisiting the hallmarks of cancer: the role of hyaluronan. Semin. Cancer Biol. 62, 9–19 (2020).
Tavianatou, A. G. et al. Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 286, 2883–2908 (2019).
Roycik, M. D., Fang, X. & Sang, Q. X. A fresh prospect of extracellular matrix hydrolytic enzymes and their substrates. Curr. Pharm. Des. 15, 1295–1308 (2009).
Myllyharju, J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 22, 15–24 (2003).
Qi, Y. & Xu, R. Roles of plods in collagen synthesis and cancer progression. Front. Cell Dev. Biol. 6, 66 (2018).
Barker, H. E., Cox, T. R. & Erler, J. T. The rationale for targeting the LOX family in cancer. Nat. Rev. Cancer 12, 540–552 (2012).
Yuzhalin, A. E., Lim, S. Y., Kutikhin, A. G. & Gordon-Weeks, A. N. Dynamic matrisome: ECM remodeling factors licensing cancer progression and metastasis. Biochim. Biophys. Acta Rev. Cancer 1870, 207–228 (2018).
Hammond, E., Khurana, A., Shridhar, V. & Dredge, K. The role of heparanase and sulfatases in the modification of heparan sulfate proteoglycans within the tumor microenvironment and opportunities for novel cancer therapeutics. Front. Oncol. 4, 195 (2014).
Coombe, D. R. & Gandhi, N. S. Heparanase: a challenging cancer drug target. Front. Oncol. 9, 1316 (2019).
Fonović, M. & Turk, B. Cysteine cathepsins and extracellular matrix degradation. Biochim. Biophys. Acta 1840, 2560–2570 (2014).
Kessenbrock, K., Wang, C.-Y. & Werb, Z. Matrix metalloproteinases in stem cell regulation and cancer. Matrix Biol. 44–46, 184–190 (2015).
Pires, A. et al. Immune remodelling of the extracellular matrix drives loss of cancer stem cells and tumor rejection. Cancer Immunol. Res. https://doi.org/10.1158/2326-6066.CIR-20-0070 (2020). Study on the interplay between the tumour matrix and the immune response, and in particular matrix-remodelling effects on the elimination of cancer stem cells, and propagation of adaptive immunity.
Filipe, E. C., Chitty, J. L. & Cox, T. R. Charting the unexplored extracellular matrix in cancer. Int. J. Exp. Pathol. 99, 58–76 (2018).
Nam, S., Hu, K. H., Butte, M. J. & Chaudhuri, O. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc. Natl Acad. Sci. USA 113, 5492–5497 (2016).
Chaudhuri, O., Cooper-White, J., Janmey, P. A., Mooney, D. J. & Shenoy, V. B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020). Comprehensive review of the complex mechanical behaviours of tissues and extracellular matrices, and the effects that matrix viscoelasticity has on cells.
Chitty, J. L., Setargew, Y. F. I. & Cox, T. R. Targeting the lysyl oxidases in tumour desmoplasia. Biochem. Soc. Trans. 47, 1661–1678 (2019).
El-Haibi, C. P. et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc. Natl Acad. Sci. USA 109, 17460–17465 (2012).
Chu, I. M. et al. GATA3 inhibits lysyl oxidase-mediated metastases of human basal triple-negative breast cancer cells. Oncogene 31, 2017–2027 (2012).
Taylor, M. A., Amin, J. D., Kirschmann, D. A. & Schiemann, W. P. Lysyl oxidase contributes to mechanotransduction-mediated regulation of transforming growth factor-β signaling in breast cancer cells. Neoplasia 13, 406–418 (2011).
Cox, T. R. et al. The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522, 106–110 (2015).
Erler, J. T. et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44 (2009).
Pickup, M. W. et al. Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-β-deficient mouse mammary carcinomas. Cancer Res. 73, 5336–5346 (2013).
Levental, K. R. et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139, 891–906 (2009). Study on how collagen crosslinking by LOX stiffens the extracellular matrix, leading to activation of intracellular signalling that drives cell invasion in premalignant epithelial cells.
Reynaud, C. et al. Lysyl oxidase is a strong determinant of tumor cell colonization in bone. Cancer Res. 77, 268–278 (2017).
Baker, A. M., Bird, D., Lang, G., Cox, T. R. & Erler, J. T. Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene 32, 1863–1868 (2013).
Baker, A.-M. et al. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J. Natl Cancer Inst. 103, 407–424 (2011).
Baker, A.-M. et al. Lysyl oxidase plays a critical role in endothelial cell stimulation to drive tumor angiogenesis. Cancer Res. 73, 583–594 (2013).
Le Calvé, B. et al. Lysyl oxidase family activity promotes resistance of pancreatic ductal adenocarcinoma to chemotherapy by limiting the intratumoral anticancer drug distribution. Oncotarget 7, 32100–32112 (2016).
DuFort, C. C., DelGiorno, K. E. & Hingorani, S. R. Mounting pressure in the microenvironment: fluids, solids, and cells in pancreatic ductal adenocarcinoma. Gastroenterology 150, 1545–1557.e2 (2016).
Scarpellini, A. et al. Heparan sulfate proteoglycans are receptors for the cell-surface trafficking and biological activity of transglutaminase-2. J. Biol. Chem. 284, 18411–18423 (2009).
Barsigian, C., Fellin, F. M., Jain, A. & Martinez, J. Dissociation of fibrinogen and fibronectin binding from transglutaminase-mediated cross-linking at the hepatocyte surface. J. Biol. Chem. 263, 14015–14022 (1988).
Cardoso, I. et al. Transglutaminase 2 interactions with extracellular matrix proteins as probed with coeliac disease autoantibodies. FEBS J. 282, 2063–2075 (2015).
Akimov, S. S., Krylov, D., Fleischman, L. F. & Belkin, A. M. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J. Cell Biol. 148, 825–838 (2000).
Shinde, A. et al. Transglutaminase-2 facilitates extracellular vesicle-mediated establishment of the metastatic niche. Oncogenesis 9, 16 (2020).
Rouhiainen, A., Kuja-Panula, J., Tumova, S. & Rauvala, H. RAGE-mediated cell signaling. Methods Mol. Biol. 963, 239–263 (2013).
Ahmad, S. et al. AGEs, RAGEs and s-RAGE; friend or foe for cancer. Semin. Cancer Biol. 49, 44–55 (2018).
Haque, E. et al. Advanced glycation end products (AGEs), protein aggregation and their crosstalk: new insight in tumorigenesis. Glycobiology https://doi.org/10.1093/glycob/cwz073 (2019).
Bergers, G. et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2, 737–744 (2000).
Nagase, H., Visse, R. & Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 69, 562–573 (2006).
Kessenbrock, K., Plaks, V. & Werb, Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52–67 (2010).
Shimoda, M., Ohtsuka, T., Okada, Y. & Kanai, Y. Stromal metalloproteinases: Crucial contributors to the tumor microenvironment. Pathol. Int. https://doi.org/10.1111/pin.13033 (2020).
Winkler, J., Abisoye-Ogunniyan, A., Metcalf, K. J. & Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 11, 5120 (2020).
Åström, P. et al. The interplay of matrix metalloproteinase-8, transforming growth factor-β1 and vascular endothelial growth factor-C cooperatively contributes to the aggressiveness of oral tongue squamous cell carcinoma. Br. J. Cancer 117, 1007–1016 (2017).
Stadlmann, S. et al. Cytokine-regulated expression of collagenase-2 (MMP-8) is involved in the progression of ovarian cancer. Eur. J. Cancer 39, 2499–2505 (2003).
Qin, G. et al. Reciprocal activation between MMP-8 and TGF-β1 stimulates EMT and malignant progression of hepatocellular carcinoma. Cancer Lett. 374, 85–95 (2016).
Raeeszadeh-Sarmazdeh, M., Do, L. D. & Hritz, B. G. Metalloproteinases and their inhibitors: potential for the development of new therapeutics. Cells 9, 1313 (2020).
Bonnans, C., Chou, J. & Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 15, 786–801 (2014).
Rossello, A., Nuti, E., Ferrini, S. & Fabbi, M. Targeting ADAM17 sheddase activity in cancer. Curr. Drug Targets 17, 1908–1927 (2016).
Buck, M. R., Karustis, D. G., Day, N. A., Honn, K. V. & Sloane, B. F. Degradation of extracellular-matrix proteins by human cathepsin B from normal and tumour tissues. Biochem. J. 282, 273–278 (1992).
Ishidoh, K. & Kominami, E. Procathepsin L degrades extracellular matrix proteins in the presence of glycosaminoglycans in vitro. Biochem. Biophys. Res. Commun. 217, 624–631 (1995).
Taleb, S., Cancello, R., Clément, K. & Lacasa, D. Cathepsin s promotes human preadipocyte differentiation: possible involvement of fibronectin degradation. Endocrinology 147, 4950–4959 (2006).
Mai, J., Sameni, M., Mikkelsen, T. & Sloane, B. F. Degradation of extracellular matrix protein tenascin-C by cathepsin B: an interaction involved in the progression of gliomas. Biol. Chem. 383, 1407–1413 (2002).
Sage, J. et al. Cleavage of nidogen-1 by cathepsin S impairs its binding to basement membrane partners. PLoS ONE 7, e43494 (2012).
Olson, O. C. & Joyce, J. A. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat. Rev. Cancer 15, 712–729 (2015).
Vadon-Le Goff, S., Hulmes, D. J. S. & Moali, C. BMP-1/tolloid-like proteinases synchronize matrix assembly with growth factor activation to promote morphogenesis and tissue remodeling. Matrix Biol. 44–46, 14–23 (2015).
Torres, S. et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin. Cancer Res. 19, 6006–6019 (2013).
Wu, X. et al. miR-194 suppresses metastasis of non-small cell lung cancer through regulating expression of BMP1 and p27(kip1). Oncogene 33, 1506–1514 (2014).
Stern, R. Hyaluronidases in cancer biology. Semin. Cancer Biol. 18, 275–280 (2008).
Liu, M., Tolg, C. & Turley, E. Dissecting the dual nature of hyaluronan in the tumor microenvironment. Front. Immunol. 10, 947 (2019).
Yamaguchi, Y., Yamamoto, H., Tobisawa, Y. & Irie, F. TMEM2: A missing link in hyaluronan catabolism identified? Matrix Biol. 78–79, 139–146 (2019).
Tammi, M. I. et al. Activated hyaluronan metabolism in the tumor matrix - causes and consequences. Matrix Biol. 78–79, 147–164 (2019).
Jacobetz, M. A. et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120 (2013).
Bame, K. J. Heparanases: endoglycosidases that degrade heparan sulfate proteoglycans. Glycobiology 11, 91R–98R (2001).
Sanderson, R. D., Elkin, M., Rapraeger, A. C., Ilan, N. & Vlodavsky, I. Heparanase regulation of cancer, autophagy and inflammation: new mechanisms and targets for therapy. FEBS J. 284, 42–55 (2017).
Khanna, M. & Parish, C. R. Heparanase: historical aspects and future perspectives. Adv. Exp. Med. Biol. 1221, 71–96 (2020).
Elgundi, Z. et al. Cancer metastasis: the role of the extracellular matrix and the heparan sulfate proteoglycan perlecan. Front. Oncol. 9, 1482 (2019).
Vlodavsky, I. et al. Significance of heparanase in cancer and inflammation. Cancer Microenviron. 5, 115–132 (2012).
Masola, V., Bellin, G., Gambaro, G. & Onisto, M. Heparanase: a multitasking protein involved in extracellular matrix (ECM) remodeling and intracellular events. Cells 7, 236 (2018).
Vlodavsky, I., Gross-Cohen, M., Weissmann, M., Ilan, N. & Sanderson, R. D. Opposing functions of heparanase-1 and heparanase-2 in cancer progression. Trends Biochem. Sci. 43, 18–31 (2018).
Roy, M. & Marchetti, D. Cell surface heparan sulfate released by heparanase promotes melanoma cell migration and angiogenesis. J. Cell Biochem. 106, 200–209 (2009).
Brew, K. & Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim. Biophys. Acta 1803, 55–71 (2010).
Jackson, H. W., Defamie, V., Waterhouse, P. & Khokha, R. TIMPs: versatile extracellular regulators in cancer. Nat. Rev. Cancer 17, 38–53 (2017).
Dechaphunkul, A. et al. Prognostic significance of tissue inhibitor of metalloproteinase-1 in breast cancer. Int. J. Breast Cancer 2012, 290854 (2012).
Ring, P., Johansson, K., Höyhtyä, M., Rubin, K. & Lindmark, G. Expression of tissue inhibitor of metalloproteinases TIMP-2 in human colorectal cancer–a predictor of tumour stage. Br. J. Cancer 76, 805–811 (1997).
Grünwald, B. et al. Pancreatic premalignant lesions secrete tissue inhibitor of metalloproteinases-1, which activates hepatic stellate cells Via CD63 signaling to create a premetastatic niche in the liver. Gastroenterology 151, 1011–1024.e7 (2016).
Breznik, B., Mitrović, A., T Lah, T. & Kos, J. Cystatins in cancer progression: More than just cathepsin inhibitors. Biochimie 166, 233–250 (2019).
Tian, C. et al. Cancer cell-derived matrisome proteins promote metastasis in pancreatic ductal adenocarcinoma. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-19-2578 (2020).
Valiente, M. et al. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell 156, 1002–1016 (2014).
Becerra, S. P. & Notario, V. The effects of PEDF on cancer biology: mechanisms of action and therapeutic potential. Nat. Rev. Cancer 13, 258–271 (2013).
Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10, 712–723 (2010).
Ricard-Blum, S. & Vallet, S. D. Proteases decode the extracellular matrix cryptome. Biochimie 122, 300–313 (2016).
Lee, J. H. et al. Endostatin: a novel inhibitor of androgen receptor function in prostate cancer. Proc. Natl Acad. Sci. USA 112, 1392–1397 (2015).
Magnon, C. et al. Canstatin acts on endothelial and tumor cells via mitochondrial damage initiated through interaction with alphavbeta3 and alphavbeta5 integrins. Cancer Res. 65, 4353–4361 (2005).
Wang, S. et al. Endostatin has ATPase activity, which mediates its antiangiogenic and antitumor activities. Mol. Cancer Ther. 14, 1192–1201 (2015).
Colorado, P. C. et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 60, 2520–2526 (2000).
Maeshima, Y., Colorado, P. C. & Kalluri, R. Two RGD-independent alpha vbeta 3 integrin binding sites on tumstatin regulate distinct anti-tumor properties. J. Biol. Chem. 275, 23745–23750 (2000).
Albrengues, J. et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science 361, eaao4227 (2018). Study that sheds new, unexpected light on the mechanisms involved in neutrophil-mediated tumour promotion through remodelling of the extracellular matrix.
Parker, A. L. & Cox, T. R. The role of the ECM in lung cancer dormancy and outgrowth. Front. Oncol. 10, 1766 (2020).
Liu, T., Zhou, L., Li, D., Andl, T. & Zhang, Y. Cancer-associated fibroblasts build and secure the tumor microenvironment. Front. Cell Dev. Biol. 7, 60 (2019).
Foster, D. S., Jones, R. E., Ransom, R. C., Longaker, M. T. & Norton, J. A. The evolving relationship of wound healing and tumor stroma. JCI Insight 3, e99911 (2018).
Dvorak, H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 (1986). Early work discussing the similarities between the extracellular matrix in tumours and wound healing.
Dvorak, H. F. Tumors: wounds that do not heal-a historical perspective with a focus on the fundamental roles of increased vascular permeability and clotting. Semin. Thromb. Hemost. 45, 576–592 (2019).
Pereira, B. A. et al. CAF subpopulations: a new reservoir of stromal targets in pancreatic cancer. Trends Cancer 5, 724–741 (2019).
Bartoschek, M. et al. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat. Commun. 9, 5150 (2018).
Nguyen, E. V. et al. Proteomic profiling of human prostate cancer-associated fibroblasts (CAF) reveals LOXL2-dependent regulation of the tumor microenvironment. Mol. Cell Proteom. 18, 1410–1427 (2019).
Sahai, E. et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020). A consensus framework for the identification and study of CAFs and their roles in cancer.
LeBleu, V. S. & Kalluri, R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis. Model. Mech. 11, dmm029447 (2018).
Arina, A. et al. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl Acad. Sci. USA 113, 7551–7556 (2016).
Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 16, 582–598 (2016). Comprehensive review of the biology and function of fibroblasts in solid tumours.
Bochet, L. et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 73, 5657–5668 (2013).
Mu, W., Rana, S. & Zöller, M. Host matrix modulation by tumor exosomes promotes motility and invasiveness. Neoplasia 15, 875–887 (2013).
Webber, J. P. et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene 34, 290–302 (2015).
LeBleu, V. S. & Kalluri, R. Exosomes as a multicomponent biomarker platform in cancer. Trends Cancer 6, 767–774 (2020).
Liu, L. et al. Stromal myofibroblasts are associated with poor prognosis in solid cancers: a meta-analysis of published studies. PLoS ONE 11, e0159947 (2016).
Öhlund, D. et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214, 579–596 (2017).
Elyada, E. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123 (2019).
Ishimoto, T. et al. Activation of transforming growth factor beta 1 signaling in gastric cancer-associated fibroblasts increases their motility, via expression of rhomboid 5 homolog 2, and ability to induce invasiveness of gastric cancer cells. Gastroenterology 153, 191–204.e16 (2017).
Vasiukov, G. et al. Myeloid cell-derived TGF-beta signaling regulates ECM deposition in mammary carcinoma via adenosine-dependent mechanisms. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-19-3954 (2020).
Biffi, G. et al. IL1-Induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 9, 282–301 (2019).
Hiebert, P. et al. Nrf2-mediated fibroblast reprogramming drives cellular senescence by targeting the matrisome. Dev. Cell 46, 145–161.e10 (2018).
Le, C. P. et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 7, 10634 (2016).
Nagaraja, A. S. et al. Adrenergic-mediated increases in INHBA drive CAF phenotype and collagens. JCI Insight 2, e93076 (2017).
Insua-Rodríguez, J. et al. Stress signaling in breast cancer cells induces matrix components that promote chemoresistant metastasis. EMBO Mol. Med. 10, e9003 (2018).
Coffey, J. C. et al. Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 4, 760–768 (2003).
Rachman-Tzemah, C. et al. Blocking surgically induced lysyl oxidase activity reduces the risk of lung metastases. Cell Rep. 19, 774–784 (2017).
Steins, A. et al. High-grade mesenchymal pancreatic ductal adenocarcinoma drives stromal deactivation through CSF-1. EMBO Rep. 21, e48780 (2020).
Özdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014).
Rhim, A. D. et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014).
Amakye, D., Jagani, Z. & Dorsch, M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat. Med. 19, 1410–1422 (2013).
Cox, T. R. & Erler, J. T. Fibrosis and cancer: partners in crime or opposing forces? Trends Cancer 2, 279–282 (2016).
Wei, L. et al. Cancer-associated fibroblasts promote progression and gemcitabine resistance via the SDF-1/SATB-1 pathway in pancreatic cancer. Cell Death Dis. 9, 1065 (2018).
Hastings, J. F., Skhinas, J. N., Fey, D., Croucher, D. R. & Cox, T. R. The extracellular matrix as a key regulator of intracellular signalling networks. Br. J. Pharmacol. 176, 82–92 (2019).
Ecker, B. L. et al. Age-related changes in HAPLN1 increase lymphatic permeability and affect routes of melanoma metastasis. Cancer Discov. 9, 82–95 (2019). Study highlighting how age-related changes in HAPLN1 in draining lymph nodes affect sites of metastasis in melanoma.
Kaur, A. et al. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 9, 64–81 (2019). Study investigating how age-related changes in the skin, and in particular HAPLN1, alter response to immunotherapy and metastatic dissemination.
Chaudhuri, O. et al. Substrate stress relaxation regulates cell spreading. Nat. Commun. 6, 6364 (2015).
Guo, W. et al. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell 126, 489–502 (2006).
Mocanu, M.-M. et al. Associations of ErbB2, beta1-integrin and lipid rafts on Herceptin (Trastuzumab) resistant and sensitive tumor cell lines. Cancer Lett. 227, 201–212 (2005).
Grasset, E. M. et al. Matrix stiffening and EGFR cooperate to promote the collective invasion of cancer cells. Cancer Res. 78, 5229–5242 (2018).
Weigelt, B., Lo, A. T., Park, C. C., Gray, J. W. & Bissell, M. J. HER2 signaling pathway activation and response of breast cancer cells to HER2-targeting agents is dependent strongly on the 3D microenvironment. Breast Cancer Res. Treat. 122, 35–43 (2010).
Zanconato, F., Cordenonsi, M. & Piccolo, S. YAP and TAZ: a signalling hub of the tumour microenvironment. Nat. Rev. Cancer 19, 454–464 (2019).
Nguyen, C. D. K. & Yi, C. YAP/TAZ signaling and resistance to cancer therapy. Trends Cancer 5, 283–296 (2019).
Vennin, C. et al. Transient tissue priming via ROCK inhibition uncouples pancreatic cancer progression, sensitivity to chemotherapy, and metastasis. Sci. Transl. Med. 9, eaai8504 (2017). Study demonstrating how inhibition of ROCK signalling in tumours disrupts matrix remodelling, leading to decreased metastasis and increased response to therapy in pancreatic cancer models.
Rath, N. et al. ROCK signaling promotes collagen remodeling to facilitate invasive pancreatic ductal adenocarcinoma tumor cell growth. EMBO Mol. Med. 9, 198–218 (2017).
Rath, N. et al. Rho kinase inhibition by AT13148 blocks pancreatic ductal adenocarinoma invasion and tumor growth. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-17-1339 (2018).
Ibbetson, S. J., Pyne, N. T., Pollard, A. N., Olson, M. F. & Samuel, M. S. Mechanotransduction pathways promoting tumor progression are activated in invasive human squamous cell carcinoma. Am. J. Pathol. 183, 930–937 (2013).
Boyle, S. T. et al. ROCK-mediated selective activation of PERK signalling causes fibroblast reprogramming and tumour progression through a CRELD2-dependent mechanism. Nat. Cell Biol. 22, 882–895 (2020). Study in breast cancer models revealing how cancer cell-driven CAF reprograming leads to the generation of a protumorigenic matrix.
Vennin, C. et al. Reshaping the tumor stroma for treatment of pancreatic cancer. Gastroenterology 154, 820–838 (2018).
Kechagia, J. Z., Ivaska, J. & Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 20, 457–473 (2019).
Hamidi, H. & Ivaska, J. Every step of the way: integrins in cancer progression and metastasis. Nat. Rev. Cancer 18, 533–548 (2018).
Franco-Barraza, J. et al. Matrix-regulated integrin αvβ5 maintains α5β1-dependent desmoplastic traits prognostic of neoplastic recurrence. eLife 6, e20600 (2017).
Samaržija, I. et al. Integrin crosstalk contributes to the complexity of signalling and unpredictable cancer cell fates. Cancers 12, 1910 (2020).
Madamanchi, A., Zijlstra, A. & Zutter, M. M. Flipping the switch: integrin switching provides metastatic competence. Sci. Signal. 7, pe9 (2014).
Young, J. L. et al. Integrin subtypes and nanoscale ligand presentation influence drug sensitivity in cancer cells. Nano Lett. 20, 1183–1191 (2020).
Kuninty, P. R. et al. ITGA5 inhibition in pancreatic stellate cells attenuates desmoplasia and potentiates efficacy of chemotherapy in pancreatic cancer. Sci. Adv. 5, eaax2770 (2019).
Wheelock, M. J., Shintani, Y., Maeda, M., Fukumoto, Y. & Johnson, K. R. Cadherin switching. J. Cell Sci. 121, 727–735 (2008).
Janiszewska, M., Primi, M. C. & Izard, T. Cell adhesion in cancer: beyond the migration of single cells. J. Biol. Chem. 295, 2495–2505 (2020).
Zuidema, A., Wang, W. & Sonnenberg, A. Crosstalk between cell adhesion complexes in regulation of mechanotransduction. Bioessays https://doi.org/10.1002/bies.202000119 (2020).
Valiathan, R. R., Marco, M., Leitinger, B., Kleer, C. G. & Fridman, R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 31, 295–321 (2012).
Takai, K. et al. Discoidin domain receptor 1 (DDR1) ablation promotes tissue fibrosis and hypoxia to induce aggressive basal-like breast cancers. Genes Dev. 32, 244–257 (2018).
Gonzalez, M. E. et al. Mesenchymal stem cell-induced ddr2 mediates stromal-breast cancer interactions and metastasis growth. Cell Rep. 18, 1215–1228 (2017).
Bayer, S. V. et al. DDR2 controls breast tumor stiffness and metastasis by regulating integrin mediated mechanotransduction in CAFs. eLife 8, e45508 (2019).
Tu, M. M. et al. Targeting DDR2 enhances tumor response to anti-PD-1 immunotherapy. Sci. Adv. 5, eaav2437 (2019).
Chronopoulos, A. et al. Syndecan-4 tunes cell mechanics by activating the kindlin-integrin-RhoA pathway. Nat. Mater. 19, 669–678 (2020).
Miller, A. E., Hu, P. & Barker, T. H. Feeling things out: bidirectional signaling of the cell-ECM interface, implications in the mechanobiology of cell spreading, migration, proliferation, and differentiation. Adv. Healthc. Mater https://doi.org/10.1002/adhm.201901445 (2020).
Zanotelli, M. R., Chada, N. C., Johnson, C. A. & Reinhart-King, C. A. The physical microenvironment of tumors: characterization and clinical impact. Biophys. Rev. Lett. https://doi.org/10.1142/S1793048020300029 (2020).
Wirtz, D., Konstantopoulos, K. & Searson, P. C. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 11, 512–522 (2011).
Smith, L. R., Cho, S. & Discher, D. E. Stem cell differentiation is regulated by extracellular matrix mechanics. Physiology 33, 16–25 (2018).
Batlle, E. & Clevers, H. Cancer stem cells revisited. Nat. Med. 23, 1124–1134 (2017). Comprehensive review of the role cancer stem cells in cancer.
Hoffmann, E. J. & Ponik, S. M. Biomechanical contributions to macrophage activation in the tumor microenvironment. Front. Oncol. 10, 787 (2020).
Malandrino, A., Mak, M., Kamm, R. D. & Moeendarbary, E. Complex mechanics of the heterogeneous extracellular matrix in cancer. Extreme Mech. Lett. 21, 25–34 (2018).
Gong, Z. et al. Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proc. Natl Acad. Sci. USA 115, E2686–E2695 (2018).
Tschumperlin, D. J. & Lagares, D. Mechano-therapeutics: targeting mechanical signaling in fibrosis and tumor stroma. Pharmacol. Ther. https://doi.org/10.1016/j.pharmthera.2020.107575 (2020).
Pratt, S. J. P., Lee, R. M. & Martin, S. S. The mechanical microenvironment in breast cancer. Cancers 12, 1452 (2020).
Fox, A. H. & Lamond, A. I. Paraspeckles. Cold Spring Harb. Perspect. Biol. 2, a000687 (2010).
Todorovski, V., Fox, A. H. & Choi, Y. S. Matrix stiffness-sensitive long-non coding RNA NEAT1 seeded paraspeckles in cancer cells. Mol. Biol. Cell https://doi.org/10.1091/mbc.E20-02-0097 (2020).
Nazemi, M. & Rainero, E. Cross-talk between the tumor microenvironment, extracellular matrix, and cell metabolism in cancer. Front. Oncol. 10, 239 (2020).
Papalazarou, V. et al. The creatine-phosphagen system is mechanoresponsive in pancreatic adenocarcinoma and fuels invasion and metastasis. Nat. Metab. 2, 62–80 (2020).
Romani, P. et al. Extracellular matrix mechanical cues regulate lipid metabolism through Lipin-1 and SREBP. Nat. Cell Biol. 21, 338–347 (2019).
Park, J. S. et al. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature 578, 621–626 (2020).
Becker, L. M. et al. Epigenetic reprogramming of cancer-associated fibroblasts deregulates glucose metabolism and facilitates progression of breast cancer. Cell Rep. 31, 107701 (2020).
Bertero, T. et al. Tumor-stroma mechanics coordinate amino acid availability to sustain tumor growth and malignancy. Cell Metab. 29, 124–140.e10 (2019).
Demircioglu, F. et al. Cancer associated fibroblast FAK regulates malignant cell metabolism. Nat. Commun. 11, 1290 (2020).
Nallanthighal, S. et al. Inhibition of collagen XI alpha 1-induced fatty acid oxidation triggers apoptotic cell death in cisplatin-resistant ovarian cancer. Cell Death Dis. 11, 258 (2020).
Slaughter, D. P., Southwick, H. W. & Smejkal, W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6, 963–968 (1953).
Ge, L., Meng, W., Zhou, H. & Bhowmick, N. Could stroma contribute to field cancerization? Med. Hypotheses 75, 26–31 (2010).
Bissell, M. J. & Hines, W. C. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 17, 320–329 (2011). Study in pancreatic cancer dissecting how the genotype of cancer cells shapes their secretomes to differentially reprogram local CAF populations, leading to remodelling of the matrix, creating pro-invasive and chemoprotective microenvironments.
Panciera, T. et al. Reprogramming normal cells into tumour precursors requires ECM stiffness and oncogene-mediated changes of cell mechanical properties. Nat. Mater. 19, 797–806 (2020).
Laklai, H. et al. Genotype tunes pancreatic ductal adenocarcinoma tissue tension to induce matricellular fibrosis and tumor progression. Nat. Med. 22, 497–505 (2016). Study on how the extracellular matrix and in particular tumour fibrosis cooperates with genetic status in pancreatic cancer to drive progression.
Vennin, C. et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat. Commun. 10, 3637 (2019). Study in pancreatic cancer dissecting how the genotype of cancer cells shapes their secretomes to differentially reprogramme local CAF populations, leading to remodelling of the matrix, creating proinvasive and chemoprotective microenvironments.
Arandkar, S. et al. Altered p53 functionality in cancer-associated fibroblasts contributes to their cancer-supporting features. Proc. Natl Acad. Sci. USA 115, 6410–6415 (2018).
Cazet, A. S. et al. Targeting stromal remodeling and cancer stem cell plasticity overcomes chemoresistance in triple negative breast cancer. Nat. Commun. 9, 2897 (2018).
Barcus, C. E. et al. Elevated collagen-I augments tumor progressive signals, intravasation and metastasis of prolactin-induced estrogen receptor alpha positive mammary tumor cells. Breast Cancer Res. 19, 9 (2017).
Welch, D. R. & Hurst, D. R. Defining the hallmarks of metastasis. Cancer Res. 79, 3011–3027 (2019). Review of the emerging concept of the hallmarks of metastasis and the role the extracellular matrix plays in these.
Conklin, M. W. et al. Collagen alignment as a predictor of recurrence after ductal carcinoma in situ. Cancer Epidemiol. Biomarkers Prev. 27, 138–145 (2018).
Mayorca-Guiliani, A. E. et al. ISDoT: in situ decellularization of tissues for high-resolution imaging and proteomic analysis of native extracellular matrix. Nat. Med. 23, 890–898 (2017).
Feinberg, T. Y. et al. Divergent matrix-remodeling strategies distinguish developmental from neoplastic mammary epithelial cell invasion programs. Dev. Cell 47, 145–160.e6 (2018).
Gao, H. et al. Multi-organ site metastatic reactivation mediated by non-canonical discoidin domain receptor 1 signaling. Cell 166, 47–62 (2016).
Elia, I. et al. Breast cancer cells rely on environmental pyruvate to shape the metastatic niche. Nature 568, 117–121 (2019).
Micalizzi, D. S., Maheswaran, S. & Haber, D. A. A conduit to metastasis: circulating tumor cell biology. Genes Dev. 31, 1827–1840 (2017).
Follain, G. et al. Fluids and their mechanics in tumour transit: shaping metastasis. Nat. Rev. Cancer 20, 107–124 (2020).
Haemmerle, M. et al. Platelets reduce anoikis and promote metastasis by activating YAP1 signaling. Nat. Commun. 8, 310 (2017).
Yu, M. et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature 487, 510–513 (2012).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017). Comprehensive landmark review of the emerging concept of premetastatic niches and their importance in metastasis and metastatic organotropism.
Gao, Y. et al. Metastasis organotropism: redefining the congenial soil. Dev. Cell 49, 375–391 (2019).
Hebert, J. D. et al. Proteomic profiling of the ECM of xenograft breast cancer metastases in different organs reveals distinct metastatic niches. Cancer Res. 80, 1475–1485 (2020).
Malanchi, I. et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature 481, 85–89 (2011).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Oskarsson, T. et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat. Med. 17, 867–874 (2011).
O’Connell, J. T. et al. VEGF-A and tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl Acad. Sci. USA 108, 16002–16007 (2011).
Cox, T. R. et al. LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res. 73, 1721–1732 (2013).
Hiratsuka, S. et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2, 289–300 (2002).
Fanjul-Fernández, M. et al. Matrix metalloproteinase Mmp-1a is dispensable for normal growth and fertility in mice and promotes lung cancer progression by modulating inflammatory responses. J. Biol. Chem. 288, 14647–14656 (2013).
Pantel, K., Alix-Panabières, C. & Riethdorf, S. Cancer micrometastases. Nat. Rev. Clin. Oncol. 6, 339–351 (2009).
Goddard, E. T., Bozic, I., Riddell, S. R. & Ghajar, C. M. Dormant tumour cells, their niches and the influence of immunity. Nat. Cell Biol. 20, 1240–1249 (2018). Review of the role the tumour microenvironment at metastatic sites and the importance this plays in disseminated tumour cell dormancy.
Yeh, A. C. & Ramaswamy, S. Mechanisms of cancer cell dormancy–another hallmark of cancer? Cancer Res. 75, 5014–5022 (2015).
Boire, A., Coffelt, S. B., Quezada, S. A., Vander Heiden, M. G. & Weeraratna, A. T. Tumour dormancy and reawakening: opportunities and challenges. Trends Cancer 5, 762–765 (2019).
Phan, T. G. & Croucher, P. I. The dormant cancer cell life cycle. Nat. Rev. Cancer 20, 398–411 (2020).
Altorki, N. K. et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat. Rev. Cancer 19, 9–31 (2019).
Gay, L. J. & Malanchi, I. The sleeping ugly: tumour microenvironment’s act to make or break the spell of dormancy. Biochim. Biophys. Acta Rev. Cancer 1868, 231–238 (2017).
Ghajar, C. M. et al. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 15, 807–817 (2013).
Carlson, P. et al. Targeting the perivascular niche sensitizes disseminated tumour cells to chemotherapy. Nat. Cell Biol. 21, 238–250 (2019).
Montagner, M. et al. Crosstalk with lung epithelial cells regulates Sfrp2-mediated latency in breast cancer dissemination. Nat. Cell Biol. 22, 289–296 (2020).
Yang, L. et al. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature 583, 133–138 (2020).
Pein, M. et al. Metastasis-initiating cells induce and exploit a fibroblast niche to fuel malignant colonization of the lungs. Nat. Commun. 11, 1494 (2020).
Barney, L. E. et al. Tumor cell-organized fibronectin maintenance of a dormant breast cancer population. Sci. Adv. 6, eaaz4157 (2020).
Shen, Y. et al. Reduction of liver metastasis stiffness improves response to bevacizumab in metastatic colorectal cancer. Cancer Cell 37, 800–817.e7 (2020).
Principe, D. R. et al. Long-term gemcitabine treatment reshapes the pancreatic tumor microenvironment and sensitizes murine carcinoma to combination immunotherapy. Cancer Res. https://doi.org/10.1158/0008-5472.CAN-19-2959 (2020).
Shen, C. J. et al. Ionizing radiation induces tumor cell lysyl oxidase secretion. BMC Cancer 14, 532 (2014).
Falou, O. et al. Evaluation of neoadjuvant chemotherapy response in women with locally advanced breast cancer using ultrasound elastography. Transl. Oncol. 6, 17–24 (2013).
Farmer, P. et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat. Med. 15, 68–74 (2009).
Erstad, D. J. et al. Fibrotic response to neoadjuvant therapy predicts survival in pancreatic cancer and is measurable with collagen-targeted molecular MRI. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-18-1359 (2020).
Sasson, A. R. et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: analysis of histopathology and outcome. Int. J. Gastrointest. Cancer 34, 121–128 (2003).
Chun, Y. S. et al. Significance of pathologic response to preoperative therapy in pancreatic cancer. Ann. Surg. Oncol. 18, 3601–3607 (2011).
Nguyen, T. V., Sleiman, M., Moriarty, T., Herrick, W. G. & Peyton, S. R. Sorafenib resistance and JNK signaling in carcinoma during extracellular matrix stiffening. Biomaterials 35, 5749–5759 (2014).
Yang, X. H. et al. Disruption of laminin-integrin-CD151-focal adhesion kinase axis sensitizes breast cancer cells to ErbB2 antagonists. Cancer Res. 70, 2256–2263 (2010).
Pupa, S. M. et al. Regulation of breast cancer response to chemotherapy by fibulin-1. Cancer Res. 67, 4271–4277 (2007).
Chakravarthy, A., Khan, L., Bensler, N. P., Bose, P. & De Carvalho, D. D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 9, 4692 (2018).
Bin Lim, S. et al. Pan-cancer analysis connects tumor matrisome to immune response. NPJ Precis. Oncol. 3, 15 (2019).
Erkan, M. et al. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin. Gastroenterol. Hepatol. 6, 1155–1161 (2008).
Troup, S. et al. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin. Cancer Res. 9, 207–214 (2003).
Li, X. et al. Prolonged exposure to extracellular lumican restrains pancreatic adenocarcinoma growth. Oncogene 36, 5432–5438 (2017).
Pearce, O. M. T. et al. Deconstruction of a metastatic tumor microenvironment reveals a common matrix response in human cancers. Cancer Discov. 8, 304–319 (2018).
Hoshino, A. et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell 182, 1044–1061.e18 (2020). Comprehensive study showing that extracellular vesicles from patients can be used as diagnostic and prognostic biomarkers, including the importance of the presence of extracellular matrix molecules within these vesicles.
Conklin, M. W. et al. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 178, 1221–1232 (2011).
Tomko, L. A. et al. Targeted matrisome analysis identifies thrombospondin-2 and tenascin-C in aligned collagen stroma from invasive breast carcinoma. Sci. Rep. 8, 12941 (2018).
McConnell, J. C. et al. Increased peri-ductal collagen micro-organization may contribute to raised mammographic density. Breast Cancer Res. 18, 5 (2016).
Northey, J. J. et al. Stiff stroma increases breast cancer risk by inducing the oncogene ZNF217. J. Clin. Invest. https://doi.org/10.1172/JCI129249 (2020).
Sage, H., Johnson, C. & Bornstein, P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J. Biol. Chem. 259, 3993–4007 (1984).
Mason, I. J., Taylor, A., Williams, J. G., Sage, H. & Hogan, B. L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell “culture shock” glycoprotein of Mr 43,000. EMBO J. 5, 1465–1472 (1986).
Jailkhani, N. et al. Noninvasive imaging of tumor progression, metastasis, and fibrosis using a nanobody targeting the extracellular matrix. Proc. Natl Acad. Sci. USA 116, 14181–14190 (2019).
Xie, Y. J. et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc. Natl Acad. Sci. USA 116, 7624–7631 (2019).
Costa, A. F., Campos, D., Reis, C. A. & Gomes, C. Targeting glycosylation: a new road for cancer drug discovery. Trends Cancer 6, 757–766 (2020).
Eder, M. et al. Bicyclic peptides as a new modality for imaging and targeting of proteins overexpressed by tumors. Cancer Res. 79, 841–852 (2019).
He, B. et al. Remodeling of metastatic vasculature reduces lung colonization and sensitizes overt metastases to immunotherapy. Cell Rep. 30, 714–724.e5 (2020).
Yeow, Y. L. et al. Immune-mediated ECM depletion improves tumour perfusion and payload delivery. EMBO Mol. Med. 11, e10923 (2019).
Ishihara, J. et al. Matrix-binding checkpoint immunotherapies enhance antitumor efficacy and reduce adverse events. Sci. Transl. Med. 9, eaan0401 (2017).
Momin, N. et al. Anchoring of intratumorally administered cytokines to collagen safely potentiates systemic cancer immunotherapy. Sci. Transl. Med. 11, eaaw2614 (2019).
Mansurov, A. et al. Collagen-binding IL-12 enhances tumour inflammation and drives the complete remission of established immunologically cold mouse tumours. Nat. Biomed. Eng. 4, 531–543 (2020).
Lingasamy, P. et al. Tumor-penetrating peptide for systemic targeting of tenascin-C. Sci. Rep. 10, 5809 (2020).
Takai, K., Le, A., Weaver, V. M. & Werb, Z. Targeting the cancer-associated fibroblasts as a treatment in triple-negative breast cancer. Oncotarget 7, 82889–82901 (2016).
Kozono, S. et al. Pirfenidone inhibits pancreatic cancer desmoplasia by regulating stellate cells. Cancer Res. 73, 2345–2356 (2013).
Charrier, A. & Brigstock, D. R. Regulation of pancreatic function by connective tissue growth factor (CTGF, CCN2). Cytokine Growth Factor. Rev. 24, 59–68 (2013).
Neesse, A. et al. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc. Natl Acad. Sci. USA 110, 12325–12330 (2013).
Froeling, F. E. M. et al. Retinoic acid-induced pancreatic stellate cell quiescence reduces paracrine Wnt-β-catenin signaling to slow tumor progression. Gastroenterology 141, 1486–97, 1497.e1 (2011).
Carapuça, E. F. et al. Anti-stromal treatment together with chemotherapy targets multiple signalling pathways in pancreatic adenocarcinoma. J. Pathol. 239, 286–296 (2016).
Chauhan, V. P. et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 4, 2516 (2013).
Murphy, J. E. et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 5, 1020–1027 (2019).
Ko, A. H. et al. A phase I study of FOLFIRINOX plus IPI-926, a hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas 45, 370–375 (2016).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Catenacci, D. V. T. et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J. Clin. Oncol. 33, 4284–4292 (2015).
Awasthi, N. & Schwarz, R. E. Profile of nintedanib in the treatment of solid tumors: the evidence to date. Onco. Targets. Ther. 8, 3691–3701 (2015).
Cox, T. R., Gartland, A. & Erler, J. T. Lysyl oxidase, a targetable secreted molecule involved in cancer metastasis. Cancer Res. 76, 188–192 (2016).
Miller, B. W. et al. Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO Mol. Med. 7, 1063–1076 (2015).
Bramhall, S. R. et al. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J. Clin. Oncol. 19, 3447–3455 (2001).
Bramhall, S. R. et al. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br. J. Cancer 87, 161–167 (2002).
Goffin, J. R. et al. Phase I trial of the matrix metalloproteinase inhibitor marimastat combined with carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Clin. Cancer Res. 11, 3417–3424 (2005).
Moore, M. J. et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 21, 3296–3302 (2003).
Ji, T. et al. Designing liposomes to suppress extracellular matrix expression to enhance drug penetration and pancreatic tumor therapy. ACS Nano 11, 8668–8678 (2017).
Hingorani, S. R. et al. HALO 202: randomized phase II Study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 36, 359–366 (2018).
Ramanathan, R. K. et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J. Clin. Oncol. 37, 1062–1069 (2019).
Cortes, E. et al. Tamoxifen mechanically reprograms the tumor microenvironment via HIF-1A and reduces cancer cell survival. EMBO Rep. 20, e46557 (2019).
Ley, K., Rivera-Nieves, J., Sandborn, W. J. & Shattil, S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat. Rev. Drug Discov. 15, 173–183 (2016).
Lee, B. Y., Timpson, P., Horvath, L. G. & Daly, R. J. FAK signaling in human cancer as a target for therapeutics. Pharmacol. Ther. 146, 132–149 (2015).
Roy-Luzarraga, M. & Hodivala-Dilke, K. Molecular pathways: endothelial cell FAK-A target for cancer treatment. Clin. Cancer Res. 22, 3718–3724 (2016).
Chaudhuri, O. et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 15, 326–334 (2016).
Kim, S.-H., Turnbull, J. & Guimond, S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 209, 139–151 (2011).
Fattet, L. et al. Matrix rigidity controls epithelial-mesenchymal plasticity and tumor metastasis via a mechanoresponsive EPHA2/LYN Complex. Dev. Cell 54, 302–316.e7 (2020).
Bachmann, M., Kukkurainen, S., Hytönen, V. P. & Wehrle-Haller, B. Cell adhesion by integrins. Physiol. Rev. 99, 1655–1699 (2019).
Houghton, A. M. Mechanistic links between COPD and lung cancer. Nat. Rev. Cancer 13, 233–245 (2013).
Michelotti, G. A., Machado, M. V. & Diehl, A. M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 10, 656–665 (2013).
Huo, C. W. et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 17, 79 (2015).
Shawky, M. S. et al. Proteoglycans: potential agents in mammographic density and the associated breast cancer risk. J. Mammary Gland Biol. Neoplasia 20, 121–131 (2015).
Mereiter, S., Balmaña, M., Campos, D., Gomes, J. & Reis, C. A. Glycosylation in the era of cancer-targeted therapy: where are we heading? Cancer Cell 36, 6–16 (2019).
Yuzhalin, A. E. et al. Colorectal cancer liver metastatic growth depends on PAD4-driven citrullination of the extracellular matrix. Nat. Commun. 9, 4783 (2018).
Stefanelli, V. L. et al. Citrullination of fibronectin alters integrin clustering and focal adhesion stability promoting stromal cell invasion. Matrix Biol. 82, 86–104 (2019).
Hawkins, C. L. & Davies, M. J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 294, 19683–19708 (2019).
Saad, F. A., Salih, E. & Glimcher, M. J. Identification of osteopontin phosphorylation sites involved in bone remodeling and inhibition of pathological calcification. J. Cell Biochem. 103, 852–856 (2008).
Yalak, G., Shiu, J.-Y., Schoen, I., Mitsi, M. & Vogel, V. Phosphorylated fibronectin enhances cell attachment and upregulates mechanical cell functions. PLoS ONE 14, e0218893 (2019).
Klement, E. & Medzihradszky, K. F. Extracellular protein phosphorylation, the neglected side of the modification. Mol. Cell Proteom. 16, 1–7 (2017).
Gilkes, D. M. et al. Collagen prolyl hydroxylases are essential for breast cancer metastasis. Cancer Res. 73, 3285–3296 (2013).
Gilkes, D. M. et al. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol. Cancer Res. 11, 456–466 (2013).
Li, L., Wang, W., Li, X. & Gao, T. Association of ECRG4 with PLK1, CDK4, PLOD1 and PLOD2 in esophageal squamous cell carcinoma. Am. J. Transl. Res. 9, 3741–3748 (2017).
Sada, M. et al. Hypoxic stellate cells of pancreatic cancer stroma regulate extracellular matrix fiber organization and cancer cell motility. Cancer Lett. 372, 210–218 (2016).
Shen, Q. et al. Barrier to autointegration factor 1, procollagen-lysine, 2-oxoglutarate 5-dioxygenase 3, and splicing factor 3b subunit 4 as early-stage cancer decision markers and drivers of hepatocellular carcinoma. Hepatology 67, 1360–1377 (2018).
Nicastri, A. et al. N-glycoprotein analysis discovers new up-regulated glycoproteins in colorectal cancer tissue. J. Proteome Res. 13, 4932–4941 (2014).
Ngo, B., Van Riper, J. M., Cantley, L. C. & Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 19, 271–282 (2019).
Soares da Costa, D., Reis, R. L. & Pashkuleva, I. Sulfation of glycosaminoglycans and its implications in human health and disorders. Annu. Rev. Biomed. Eng. 19, 1–26 (2017).
Fane, M. & Weeraratna, A. T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 20, 89–106 (2020).
Shuster, S., Black, M. M. & McVitie, E. The influence of age and sex on skin thickness, skin collagen and density. Br. J. Dermatol. 93, 639–643 (1975).
Watson, R. E. B., Gibbs, N. K., Griffiths, C. E. M. & Sherratt, M. J. Damage to skin extracellular matrix induced by UV exposure. Antioxid. Redox Signal. 21, 1063–1077 (2014).
Newton, V. L. et al. Mass spectrometry-based proteomics reveals the distinct nature of the skin proteomes of photoaged compared to intrinsically aged skin. Int. J. Cosmet. Sci. 41, 118–131 (2019).
Mierke, C. T. Mechanical cues affect migration and invasion of cells from three different directions. Front. Cell Dev. Biol. 8, 583226 (2020).
Carey, S. P. et al. Local extracellular matrix alignment directs cellular protrusion dynamics and migration through Rac1 and FAK. Integr. Biol. 8, 821–835 (2016).
Park, D. et al. Extracellular matrix anisotropy is determined by TFAP2C-dependent regulation of cell collisions. Nat. Mater. 19, 227–238 (2020).
Pakshir, P. et al. Dynamic fibroblast contractions attract remote macrophages in fibrillar collagen matrix. Nat. Commun. 10, 1850 (2019).
Attieh, Y. et al. Cancer-associated fibroblasts lead tumor invasion through integrin-β3-dependent fibronectin assembly. J. Cell Biol. 216, 3509–3520 (2017).
Labernadie, A. et al. A mechanically active heterotypic E-cadherin/N-cadherin adhesion enables fibroblasts to drive cancer cell invasion. Nat. Cell Biol. 19, 224–237 (2017). Study on how cancer cells and CAFs interact with one another and the matrix, and the importance that this plays in modulating local invasion and metastasis.
Yamada, K. M. & Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 20, 738–752 (2019).
Jamal-Hanjani, M. et al. Tracking the evolution of non-small-cell lung cancer. N. Engl. J. Med. 376, 2109–2121 (2017).
Izzi, V., Davis, M. N. & Naba, A. Pan-cancer analysis of the genomic alterations and mutations of the matrisome. Cancers 12, 2046 (2020).
Robertson, C. The extracellular matrix in breast cancer predicts prognosis through composition, splicing, and crosslinking. Exp. Cell Res. 343, 73–81 (2016).
Huang, J. et al. Enhanced osteopontin splicing regulated by RUNX2 is HDAC-dependent and induces invasive phenotypes in NSCLC cells. Cancer Cell Int. 19, 306 (2019).
Khan, Z. A. et al. EDB fibronectin and angiogenesis – a novel mechanistic pathway. Angiogenesis 8, 183–196 (2005).
Efthymiou, G. et al. Shaping up the tumor microenvironment with cellular fibronectin. Front. Oncol. 10, 641 (2020).
Naba, A. et al. The extracellular matrix: tools and insights for the “omics” era. Matrix Biol. 49, 10–24 (2016). Comprehensive review of many of the approaches, tools and resources being used to study the extracellular matrix in health and disease.
Shao, X., Taha, I. N., Clauser, K. R., Gao, Y. T. & Naba, A. MatrisomeDB: the ECM-protein knowledge database. Nucleic Acids Res. 48, D1136–D1144 (2020).
Clerc, O. et al. MatrixDB: integration of new data with a focus on glycosaminoglycan interactions. Nucleic Acids Res. 47, D376–D381 (2019).
Angel, P. M. et al. Extracellular matrix imaging of breast tissue pathologies by MALDI imaging mass spectrometry. Proteomics Clin. Appl. https://doi.org/10.1002/prca.201700152 (2018).
Briggs, M. T. et al. MALDI mass spectrometry imaging of early- and late-stage serous ovarian cancer tissue reveals stage-specific N-glycans. Proteomics https://doi.org/10.1002/pmic.201800482 (2019).
Phillips, L., Gill, A. J. & Baxter, R. C. Novel prognostic markers in triple-negative breast cancer discovered by MALDI-mass spectrometry imaging. Front. Oncol. 9, 379 (2019).
Gessel, M., Spraggins, J. M., Voziyan, P., Hudson, B. G. & Caprioli, R. M. Decellularization of intact tissue enables MALDI imaging mass spectrometry analysis of the extracellular matrix. J. Mass. Spectrom. 50, 1288–1293 (2015).
Cornett, D. S., Frappier, S. L. & Caprioli, R. M. MALDI-FTICR imaging mass spectrometry of drugs and metabolites in tissue. Anal. Chem. 80, 5648–5653 (2008).
Mayorca-Guiliani, A. E. et al. Decellularization and antibody staining of mouse tissues to map native extracellular matrix structures in 3D. Nat. Protoc. 14, 3395–3425 (2019).
Hwang, J. et al. In situ imaging of tissue remodeling with collagen hybridizing peptides. ACS Nano 11, 9825–9835 (2017).
Bennink, L. L. et al. Visualizing collagen proteolysis by peptide hybridization: from 3D cell culture to in vivo imaging. Biomaterials 183, 67–76 (2018).
Acknowledgements
The author apologizes to all colleagues whose work could not be discussed due to space limitations. T.R.C. is supported by the Australian National Health and Medical Research Council, Cancer Council NSW, Cancer Institute NSW, Love Your Sister in association with the Australian National Breast Cancer Foundation, Avner Pancreatic Cancer Foundation and Susan G. Komen.
Author information
Authors and Affiliations
Contributions
The author handled all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
T.R.C. is engaged in a non-commercial collaborative project with Pharmaxis Ltd, a pharmaceutical company with ownership of a small-molecule lysyl oxidase family-targeting pipeline.
Additional information
Peer review information
Nature Reviews Cancer thanks E. Cukierman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Australian Pancreatic Cancer Matrix Atlas: https://www.pancreaticcancer.net.au/apma/
MatrisomeDB: http://matrisomedb.pepchem.org/
Matrisome Project and Extracellular Matrix Atlas: http://matrisomeproject.mit.edu
MatrixDB: http://matrixdb.univ-lyon1.fr/
Glossary
- Supramolecular
-
An entity consisting of a complex organization of more than one building block.
- Dynamic reciprocity
-
The ongoing and bidirectional interaction between cells and their microenvironment, and in particular the extracellular matrix.
- Desmoplasia
-
The dense fibrotic tissue that forms in response to insult to a tissue. It is typically observed in and around solid tumours characterized by the excessive or abnormal deposition of extracellular matrix.
- Matrisome
-
All of the extracellular matrix proteins that can potentially be expressed by the genome of a specific organism.
- Basement membranes
-
Structures visible by light microscopy and, in addition to the basal lamina, that consist of layers that are typically secreted by cells from underlying connective tissue. Many basement membranes are rich in fibronectin.
- Basal lamina
-
A molecularly defined part of the basement membrane comprising an electron-dense layer, ~20–100 nm thick, that consists of collagen IV and laminin, only visible by electron microscopy. It is made and maintained by the cells that sit on it, acting as the critical point of attachment.
- Matreotype
-
The specific, acute state of matrix composition (and/or modification) at a given point, associated with, or causal for, a given physiological condition or phenotype.
- Glycosaminoglycan
-
Also known as mucopolysaccharides, glycosaminoglycans are the most abundant heteropolysaccharide in the body. They are complex linear polysaccharides consisting of repeated alternating units of uronic acid and glycosamines.
- Matricryptins
-
Also known as matrikines or cryptikines, these are biologically active fragments of matrix molecules that have undergone limited enzymatic cleavage and have a biological activity different from that of the parent protein.
- Metzincin superfamily
-
The main endopeptidases responsible for matrix degradation, comprising matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase proteins (ADAMs) and ADAMs with thrombospondin motifs (ADAMTSs).
- Schiff base adduct
-
A subclass of imines with the general structure R2C=NR′.
- Amadori rearrangement
-
Important in carbohydrate biology, this rearrangement is the isomerization event whereby the N-glycoside of an aldose sugar is converted to the corresponding ketone by acid or base catalysis.
- Mechanotransduction
-
A form of sensory transduction in which cells convert mechanical stimuli into biological signals and vice versa.
- Endopeptidases
-
Peptidases that cleave peptide bonds of non-terminal amino acids within polypeptide chains and proteins (exopeptidases cleave only the terminal peptide bond of polypeptide chains and proteins).
- Anisotropy
-
The property of being directionally dependent, whereby a particular characteristic (such as physical or mechanical properties) varies depending on the direction of measurement.
- Vascular co-option
-
The process by which tumours hijack the vasculature of existing tissues of organs to obtain a blood supply independently of angiogenesis.
- Exosomes
-
Extracellular vesicles, typically 30–150 nm in diameter, that are secreted by all cells, including cancer cells, and contain biological molecules, including DNA, RNA and proteins.
- Viscoelasticity
-
A time-dependent response to loading or deformation.
- Field cancerization
-
The process by which areas of tissue exhibit intracellular or extracellular procarcinogenic changes that lead to areas of premalignant cells or protumorigenic matrix, respectively.
- Premetastatic niches
-
Specific microenvironments that are systemically induced within a secondary organ and thought to be important for overt colonization by metastasizing primary tumour cells.
- Neutrophil extracellular traps
-
(NETs). Complex networks of extracellular fibres that are primarily composed of chromosomal DNA and histones, and have important roles in thrombosis, inflammation and cancer.
- Neoadjuvant
-
Used to describe interventions given before a main treatment, or in the case of solid tumours, before surgery.
- Elastography
-
A non-invasive medical imaging modality that maps the elastic properties and stiffness of tissues, and is predominantly used to characterize the biomechanical properties of soft tissues.
- Vascular patency
-
The degree to which blood vessels of the vasculature are open and not blocked or obstructed.
- Basket trials
-
Clinical trials in which many tumour types carrying the same molecular or genetic aberration are grouped together and given the same treatment.
Rights and permissions
About this article
Cite this article
Cox, T.R. The matrix in cancer. Nat Rev Cancer 21, 217–238 (2021). https://doi.org/10.1038/s41568-020-00329-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-020-00329-7
This article is cited by
-
Targeting triple negative breast cancer stem cells using nanocarriers
Discover Nano (2024)
-
Prognostic value and immunological role of PD-L1 gene in pan-cancer
BMC Cancer (2024)
-
Matrix stiffness affects tumor-associated macrophage functional polarization and its potential in tumor therapy
Journal of Translational Medicine (2024)
-
The progressive trend of modeling and drug screening systems of breast cancer bone metastasis
Journal of Biological Engineering (2024)
-
Oncoprotein SET-associated transcription factor ZBTB11 triggers lung cancer metastasis
Nature Communications (2024)