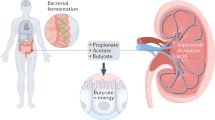
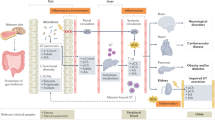
Abstract
The recognition that intestinal microbiota exert profound effects on human health has led to major advances in our understanding of disease processes. Studies over the past 20 years have shown that host components, including components of the host immune system, shape the microbial community. Pathogenic alterations in commensal microorganisms contribute to disease manifestations that are generally considered to be noncommunicable, such as inflammatory bowel disease, diabetes mellitus and liver disease, through a variety of mechanisms, including effects on host immunity. More recent studies have shed new light on how the immune system and microbiota might also drive the pathogenesis of renal disorders. In this Review, we discuss the latest insights into the mechanisms regulating the microbiome composition, with a focus both on genetics and environmental factors, and describe how commensal microorganisms calibrate innate and adaptive immune responses to affect the activation threshold for pathogenic stimulations. We discuss the mechanisms that lead to intestinal epithelial barrier inflammation and the relevance of certain bacteria to the pathogenesis of two common kidney-based disorders: hypertension and renal stone disease. Limitations of current approaches to microbiota research are also highlighted, emphasizing the need to move beyond studies of correlation to causation.
Key points
-
The gut microbiota influences the host through reciprocal interactions with the host immune system.
-
Imbalances in the microbiota (dysbiosis) lead to epithelial barrier dysfunction and translocation of toxic compounds to the systemic circulation.
-
Hypertension and kidney stone disease might be linked to compositional or functional changes in the gut microbiome.
-
Novel research methods are being established to identify causal microorganisms that drive disease progression.
-
Understanding the crosstalk between immunity, the microbiota and the kidney has potential for the development of innovative, paradigm-shifting treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Levin, A. et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 390, 1888–1917 (2017).
Thompson, S. et al. Cause of death in patients with reduced kidney function. J. Am. Soc. Nephrol. 26, 2504–2511 (2015).
Jha, V. et al. Understanding kidney care needs and implementation strategies in low- and middle-income countries: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 90, 1164–1174 (2016).
Abboud, H. & Henrich, W. L. Clinical practice. Stage IV chronic kidney disease. N. Engl. J. Med. 362, 56–65 (2010).
Forslund, K. et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528, 262–266 (2015).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012).
Dejea, C. M. et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597 (2018).
Zheng, P. et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 21, 786–796 (2016).
The Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project: dynamic analysis of microbiome-host omics profiles during periods of human health and disease. Cell Host Microbe 16, 276–289 (2014).
Kuntz, T. M. & Gilbert, J. A. Introducing the microbiome into precision medicine. Trends Pharmacol. Sci. 38, 81–91 (2017).
Zou, J. et al. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 23, 41–53 (2018).
Chung, H. et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149, 1578–1593 (2012).
Mao, K. et al. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 554, 255–259 (2018).
Moghadamrad, S. et al. Attenuated portal hypertension in germ-free mice: Function of bacterial flora on the development of mesenteric lymphatic and blood vessels. Hepatology 61, 1685–1695 (2015).
Geuking, M. B. et al. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity 34, 794–806 (2011).
Hapfelmeier, S. et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 328, 1705–1709 (2010).
Haber, A. L. et al. A single-cell survey of the small intestinal epithelium. Nature 551, 333–339 (2017).
Wiedermann, C. J. et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the Bruneck study. J. Am. Coll. Cardiol. 34, 1975–1981 (1999).
McIntyre, C. W. et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 6, 133–141 (2011).
Poesen, R. et al. Associations of soluble CD14 and endotoxin with mortality, cardiovascular disease, and progression of kidney disease among patients with CKD. Clin. J. Am. Soc. Nephrol. 10, 1525–1533 (2015).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63 (2011).
Sender, R., Fuchs, S. & Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLOS Biol. 14, e1002533 (2016).
Gill, S. R. et al. Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359 (2006).
Koenig, J. E. et al. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4578–4585 (2011).
Stappenbeck, T. S. & Virgin, H. W. Accounting for reciprocal host-microbiome interactions in experimental science. Nature 534, 191–199 (2016).
Azad, M. B. et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin. Immunol. 9, 15 (2013).
Hasegawa, K. et al. Household siblings and nasal and fecal microbiota in infants. Pediatr. Int. 59, 473–481 (2017).
Shukla, S. K. et al. The nasal microbiota of dairy farmers is more complex than oral microbiota, reflects occupational exposure, and provides competition for staphylococci. PLOS ONE 12, e0183898 (2017).
Fall, T. et al. Early exposure to dogs and farm animals and the risk of childhood asthma. JAMA Pediatr. 169, e153219 (2015).
Strachan, D. P. Hay fever, hygiene, and household size. BMJ 299, 1259–1260 (1989).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Sonnenburg, E. D. et al. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215 (2016).
Suez, J. et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 514, 181–186 (2014).
Chassaing, B. et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519, 92–96 (2015).
Gum, J. R. et al. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J. Biol. Chem. 269, 2440–2446 (1994).
Atuma, C., Strugala, V., Allen, A. & Holm, L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G922–G929 (2001).
Johansson, M. E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008).
Kim, J. J. & Khan, W. I. Goblet cells and mucins: role in innate defense in enteric infections. Pathogens 2, 55–70 (2013).
Birchenough, G. M., Nystrom, E. E., Johansson, M. E. & Hansson, G. C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 352, 1535–1542 (2016).
Wlodarska, M. et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014).
Nowarski, R. et al. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163, 1444–1456 (2015).
Ragland, S. A. & Criss, A. K. From bacterial killing to immune modulation: recent insights into the functions of lysozyme. PLOS Pathog. 13, e1006512 (2017).
Cash, H. L., Whitham, C. V., Behrendt, C. L. & Hooper, L. V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 313, 1126–1130 (2006).
Qu, X. D. & Lehrer, R. I. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect. Immun. 66, 2791–2797 (1998).
Koduri, R. S. et al. Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 277, 5849–5857 (2002).
Holly, M. K. & Smith, J. G. Paneth cells during viral infection and pathogenesis. Viruses 10, 225 (2018).
Hooper, L. V., Stappenbeck, T. S., Hong, C. V. & Gordon, J. I. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 4, 269–273 (2003).
Rumio, C. et al. Degranulation of paneth cells via toll-like receptor 9. Am. J. Pathol. 165, 373–381 (2004).
Foureau, D. M. et al. TLR9-dependent induction of intestinal alpha-defensins by Toxoplasma gondii. J. Immunol. 184, 7022–7029 (2010).
Selsted, M. E. & Ouellette, A. J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6, 551–557 (2005).
Carvalho, F. A., Aitken, J. D., Vijay-Kumar, M. & Gewirtz, A. T. Toll-like receptor-gut microbiota interactions: perturb at your own risk! Annu. Rev. Physiol. 74, 177–198 (2012).
Vaishnava, S., Behrendt, C. L., Ismail, A. S., Eckmann, L. & Hooper, L. V. Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl Acad. Sci. USA 105, 20858–20863 (2008).
Shulzhenko, N. et al. Crosstalk between B lymphocytes, microbiota and the intestinal epithelium governs immunity versus metabolism in the gut. Nat. Med. 17, 1585–1593 (2011).
Palm, N. W. et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 158, 1000–1010 (2014).
Slack, E., Balmer, M. L., Fritz, J. H. & Hapfelmeier, S. Functional flexibility of intestinal IgA - broadening the fine line. Front. Immunol. 3, 100 (2012).
Pabst, O. New concepts in the generation and functions of IgA. Nat. Rev. Immunol. 12, 821–832 (2012).
Mantis, N. J. & Forbes, S. J. Secretory IgA: arresting microbial pathogens at epithelial borders. Immunol. Invest. 39, 383–406 (2010).
Lycke, N., Erlandsson, L., Ekman, L., Schon, K. & Leanderson, T. Lack of J chain inhibits the transport of gut IgA and abrogates the development of intestinal antitoxic protection. J. Immunol. 163, 913–919 (1999).
Forbes, S. J., Eschmann, M. & Mantis, N. J. Inhibition of Salmonella enterica serovar typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin A antibody. Infect. Immun. 76, 4137–4144 (2008).
Vijay-Kumar, M. et al. Deletion of TLR5 results in spontaneous colitis in mice. J. Clin. Invest. 117, 3909–3921 (2007).
Vijay-Kumar, M. et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231 (2010).
Singh, V. et al. Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell. Metab. 22, 983–996 (2015).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Seregin, S. S. et al. NLRP6 protects Il10(−/−) mice from colitis by limiting colonization of akkermansia muciniphila. Cell Rep. 19, 733–745 (2017).
Levy, M. et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163, 1428–1443 (2015).
Galvez, E. J. C., Iljazovic, A., Gronow, A., Flavell, R. & Strowig, T. Shaping of intestinal microbiota in Nlrp6- and Rag2-deficient mice depends on community structure. Cell Rep. 21, 3914–3926 (2017).
Ubeda, C. et al. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J. Exp. Med. 209, 1445–1456 (2012).
Lemire, P. et al. The NLR protein NLRP6 does not impact gut microbiota composition. Cell Rep. 21, 3653–3661 (2017).
Mamantopoulos, M. et al. Nlrp6- and ASC-dependent inflammasomes do not shape the commensal gut microbiota composition. Immunity 47, 339–348 (2017).
Wostmann, B. S., Larkin, C., Moriarty, A. & Bruckner-Kardoss, E. Dietary intake, energy metabolism, and excretory losses of adult male germfree Wistar rats. Lab. Anim. Sci. 33, 46–50 (1983).
Stecher, B. et al. Comparison of Salmonella enterica serovar Typhimurium colitis in germfree mice and mice pretreated with streptomycin. Infect. Immun. 73, 3228–3241 (2005).
Gordon, H. A. Morphological and physiological characterization of germfree life. Ann. NY Acad. Sci. 78, 208–220 (1959).
Reinhardt, C. et al. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 483, 627–631 (2012).
Surana, N. K. & Kasper, D. L. Deciphering the tete-a-tete between the microbiota and the immune system. J. Clin. Invest. 124, 4197–4203 (2014).
Mazmanian, S. K., Liu, C. H., Tzianabos, A. O. & Kasper, D. L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122, 107–118 (2005).
Ochoa-Reparaz, J. et al. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J. Immunol. 185, 4101–4108 (2010).
Ochoa-Reparaz, J. et al. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 3, 487–495 (2010).
Round, J. L. et al. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science 332, 974–977 (2011).
Mazmanian, S. K., Round, J. L. & Kasper, D. L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625 (2008).
Shen, Y. et al. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 12, 509–520 (2012).
Dasgupta, S., Erturk-Hasdemir, D., Ochoa-Reparaz, J., Reinecker, H. C. & Kasper, D. L. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe 15, 413–423 (2014).
An, D. et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 156, 123–133 (2014).
Hoverstad, T. & Midtvedt, T. Short-chain fatty acids in germfree mice and rats. J. Nutr. 116, 1772–1776 (1986).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Tan, J. K., McKenzie, C., Marino, E., Macia, L. & Mackay, C. R. Metabolite-sensing G protein-coupled receptors-facilitators of diet-related immune regulation. Annu. Rev. Immunol. 35, 371–402 (2017).
Le Poul, E. et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 (2003).
Macia, L. et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 6, 6734 (2015).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature 504, 451–455 (2013).
Park, J. et al. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8, 80–93 (2015).
Hori, S., Nomura, T. & Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 (2003).
Flannigan, K. L. & Denning, T. L. Segmented filamentous bacteria-induced immune responses: a balancing act between host protection and autoimmunity. Immunology 154, 537–546 (2018).
Atarashi, K. et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 163, 367–380 (2015).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Ivanov, I. I. et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 4, 337–349 (2008).
Lei, Z. et al. EpCAM contributes to formation of functional tight junction in the intestinal epithelium by recruiting claudin proteins. Dev. Biol. 371, 136–145 (2012).
Laukoetter, M. G. et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 204, 3067–3076 (2007).
Tanaka, H. et al. Intestinal deletion of Claudin-7 enhances paracellular organic solute flux and initiates colonic inflammation in mice. Gut 64, 1529–1538 (2015).
Michielan, A. & D’Inca, R. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm. 2015, 628157 (2015).
Zeissig, S. et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 56, 61–72 (2007).
Ma, T. Y. et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G367–G376 (2004).
Adams, R. B., Planchon, S. M. & Roche, J. K. IFN-gamma modulation of epithelial barrier function. Time course, reversibility, and site of cytokine binding. J. Immunol. 150, 2356–2363 (1993).
Al-Sadi, R., Ye, D., Dokladny, K. & Ma, T. Y. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 180, 5653–5661 (2008).
Howe, K. L., Reardon, C., Wang, A., Nazli, A. & McKay, D. M. Transforming growth factor-beta regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157:H7-induced increased permeability. Am. J. Pathol. 167, 1587–1597 (2005).
Suenaert, P. et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn’s disease. Am. J. Gastroenterol. 97, 2000–2004 (2002).
Suenaert, P. et al. Hyperresponsiveness of the mucosal barrier in Crohn’s disease is not tumor necrosis factor-dependent. Inflamm. Bowel Dis. 11, 667–673 (2005).
Martinez-Medina, M. et al. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 63, 116–124 (2014).
Thaiss, C. A. et al. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359, 1376–1383 (2018).
Sturniolo, G. C. et al. Effect of zinc supplementation on intestinal permeability in experimental colitis. J. Lab. Clin. Med. 139, 311–315 (2002).
Roy, S. K. et al. Impact of zinc supplementation on intestinal permeability in Bangladeshi children with acute diarrhoea and persistent diarrhoea syndrome. J. Pediatr. Gastroenterol. Nutr. 15, 289–296 (1992).
Sturniolo, G. C., Di Leo, V., Ferronato, A., D’Odorico, A. & D’Inca, R. Zinc supplementation tightens “leaky gut” in Crohn’s disease. Inflamm. Bowel Dis. 7, 94–98 (2001).
Alam, A. N., Sarker, S. A., Wahed, M. A., Khatun, M. & Rahaman, M. M. Enteric protein loss and intestinal permeability changes in children during acute shigellosis and after recovery: effect of zinc supplementation. Gut 35, 1707–1711 (1994).
Venkatraman, A., Ramakrishna, B. S., Pulimood, A. B., Patra, S. & Murthy, S. Increased permeability in dextran sulphate colitis in rats: time course of development and effect of butyrate. Scand. J. Gastroenterol. 35, 1053–1059 (2000).
Mariadason, J. M., Barkla, D. H. & Gibson, P. R. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am. J. Physiol. 272, G705–G712 (1997).
Peng, L., He, Z., Chen, W., Holzman, I. R. & Lin, J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr. Res. 61, 37–41 (2007).
Vernia, P. et al. Topical butyrate improves efficacy of 5-ASA in refractory distal ulcerative colitis: results of a multicentre trial. Eur. J. Clin. Invest. 33, 244–248 (2003).
Finnie, I. A., Dwarakanath, A. D., Taylor, B. A. & Rhodes, J. M. Colonic mucin synthesis is increased by sodium butyrate. Gut 36, 93–99 (1995).
Barcelo, A. et al. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut 46, 218–224 (2000).
Ulluwishewa, D. et al. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 141, 769–776 (2011).
Patel, R. M. et al. Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function. Am. J. Pathol. 180, 626–635 (2012).
Garcia Vilela, E. et al. Influence of Saccharomyces boulardii on the intestinal permeability of patients with Crohn’s disease in remission. Scand. J. Gastroenterol. 43, 842–848 (2008).
Zakostelska, Z. et al. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLOS ONE 6, e27961 (2011).
Baez, S. & Gordon, H. A. Tone and reactivity of vascular smooth muscle in germfree rat mesentery. J. Exp. Med. 134, 846–856 (1971).
Yang, T. et al. Gut dysbiosis is linked to hypertension. Hypertension 65, 1331–1340 (2015).
Adnan, S. et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genom. 49, 96–104 (2017).
Marques, F. Z. et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 135, 964–977 (2017).
Chang, A. J., Ortega, F. E., Riegler, J., Madison, D. V. & Krasnow, M. A. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 527, 240–244 (2015).
Pluznick, J. L. et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl Acad. Sci. USA 110, 4410–4415 (2013).
Nutting, C. W., Islam, S., Ye, M. H., Batlle, D. C. & Daugirdas, J. T. The vasorelaxant effects of acetate: role of adenosine, glycolysis, lyotropism, and pHi and Cai 2+. Kidney Int. 41, 166–174 (1992).
Mell, B. et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol. Genom. 47, 187–197 (2015).
Pluznick, J. L. Gut microbiota in renal physiology: focus on short-chain fatty acids and their receptors. Kidney Int. 90, 1191–1198 (2016).
Wilck, N. et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 551, 585–589 (2017).
Santisteban, M. M. et al. Hypertension-linked pathophysiological alterations in the gut. Circ. Res. 120, 312–323 (2017).
Stewart, D. C. et al. Hypertension-linked mechanical changes of rat gut. Acta Biomater. 45, 296–302 (2016).
Yoo, H. H., Kim, I. S., Yoo, D. H. & Kim, D. H. Effects of orally administered antibiotics on the bioavailability of amlodipine: gut microbiota-mediated drug interaction. J. Hypertens. 34, 156–162 (2016).
Saksena, S. et al. Upregulation of P-glycoprotein by probiotics in intestinal epithelial cells and in the dextran sulfate sodium model of colitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G1115–G1123 (2011).
Scales, C. D. et al. Prevalence of kidney stones in the United States. Eur. Urol. 62, 160–165 (2012).
Romero, V., Akpinar, H. & Assimos, D. G. Kidney stones: a global picture of prevalence, incidence, and associated risk factors. Rev. Urol. 12, e86–96 (2010).
Alexander, R. T. et al. Kidney stones and cardiovascular events: a cohort study. Clin. J. Am. Soc. Nephrol. 9, 506–512 (2014).
Kittanamongkolchai, W. et al. The changing incidence and presentation of urinary stones over 3 decades. Mayo Clin. Proc. 93, 291–299 (2018).
Zhe, M. & Hang, Z. Nephrolithiasis as a risk factor of chronic kidney disease: a meta-analysis of cohort studies with 4,770,691 participants. Urolithiasis 45, 441–448 (2016).
Ferraro, P. M. et al. History of kidney stones and the risk of coronary heart disease. JAMA 310, 408–415 (2013).
Asplin, J. R., Parks, J. H. & Coe, F. L. Dependence of upper limit of metastability on supersaturation in nephrolithiasis. Kidney Int. 52, 1602–1608 (1997).
Bergsland, K. J., Zisman, A. L., Asplin, J. R., Worcester, E. M. & Coe, F. L. Evidence for net renal tubule oxalate secretion in patients with calcium kidney stones. Am. J. Physiol. Renal Physiol. 300, F311–F318 (2011).
Coe, F. L., Worcester, E. M. & Evan, A. P. Idiopathic hypercalciuria and formation of calcium renal stones. Nat. Rev. Nephrol. 12, 519–533 (2016).
Coe, F. L., Evan, A. P., Lingeman, J. E. & Worcester, E. M. Plaque and deposits in nine human stone diseases. Urol. Res. 38, 239–247 (2010).
Ticinesi, A. et al. Understanding the gut-kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut 67, 2097–2106 (2018).
Stern, J. M. et al. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis 44, 399–407 (2016).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Kronman, M. P., Zaoutis, T. E., Haynes, K., Feng, R. & Coffin, S. E. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 130, e794–e803 (2012).
Mitre, E. et al. Association between use of acid-suppressive medications and antibiotics during infancy and allergic diseases in early childhood. JAMA Pediatr. 172, e180315 (2018).
Tasian, G. E. et al. Oral antibiotic exposure and kidney stone disease. J. Am. Soc. Nephrol. 29, 1731–1740 (2018).
Pfau, A. & Knauf, F. Update on nephrolithiasis: core curriculum 2016. Am. J. Kidney Dis. 68, 973–985 (2016).
Hatch, M. Gut microbiota and oxalate homeostasis. Ann. Transl Med. 5, 36 (2017).
Knight, J., Deora, R., Assimos, D. G. & Holmes, R. P. The genetic composition of Oxalobacter formigenes and its relationship to colonization and calcium oxalate stone disease. Urolithiasis 41, 187–196 (2013).
Al-Wahsh, I., Wu, Y. & Liebman, M. Acute probiotic ingestion reduces gastrointestinal oxalate absorption in healthy subjects. Urol. Res. 40, 191–196 (2012).
Turroni, S. et al. Oxalate-degrading activity in Bifidobacterium animalis subsp. lactis: impact of acidic conditions on the transcriptional levels of the oxalyl coenzyme A (CoA) decarboxylase and formyl-CoA transferase genes. Appl. Environ. Microbiol. 76, 5609–5620 (2010).
Mehta, M., Goldfarb, D. S. & Nazzal, L. The role of the microbiome in kidney stone formation. Int. J. Surg. 36, 607–612 (2016).
Klimesova, K., Whittamore, J. M. & Hatch, M. Bifidobacterium animalis subsp. lactis decreases urinary oxalate excretion in a mouse model of primary hyperoxaluria. Urolithiasis 43, 107–117 (2015).
Hatch, M. et al. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 69, 691–698 (2006).
Sidhu, H. et al. Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract-dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. J. Am. Soc. Nephrol. 10 (Suppl. 14), 334–340 (1999).
Hatch, M. & Freel, R. W. A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis 41, 379–384 (2013).
Hatch, M., Freel, R. W. & Vaziri, N. D. Regulatory aspects of oxalate secretion in enteric oxalate elimination. J. Am. Soc. Nephrol. 10 (Suppl. 14), 324–328 (1999).
Knauf, F. et al. Net intestinal transport of oxalate reflects passive absorption and SLC26A6-mediated secretion. J. Am. Soc. Nephrol. 22, 2247–2255 (2011).
Jiang, Z. et al. Calcium oxalate urolithiasis in mice lacking anion transporter Slc26a6. Nat. Genet. 38, 474–478 (2006).
Freel, R. W., Hatch, M., Green, M. & Soleimani, M. Ileal oxalate absorption and urinary oxalate excretion are enhanced in Slc26a6 null mice. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G719–G728 (2006).
Arvans, D. et al. Oxalobacter formigenes-derived bioactive factors stimulate oxalate transport by intestinal epithelial cells. J. Am. Soc. Nephrol. 28, 876–887 (2017).
Mulay, S. R. et al. Oxalate-induced chronic kidney disease with its uremic and cardiovascular complications in C57BL/6 mice. Am. J. Physiol. Renal Physiol. 310, F785–F795 (2016).
Knauf, F. et al. NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy. Kidney Int. 84, 895–901 (2013).
Mulay, S. R. et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1beta secretion. J. Clin. Invest. 123, 236–246 (2013).
Kim, S. M. et al. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am. J. Physiol. Renal Physiol. 308, F993–F1003 (2015).
Prencipe, G. et al. Inflammasome activation by cystine crystals: implications for the pathogenesis of cystinosis. J. Am. Soc. Nephrol. 25, 1163–1169 (2014).
Ludwig-Portugall, I. et al. An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int. 90, 525–539 (2016).
Gilbert, J. A. et al. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 535, 94–103 (2016).
Surana, N. K. & Kasper, D. L. Moving beyond microbiome-wide associations to causal microbe identification. Nature 552, 244–247 (2017).
Rosen, C. E. & Palm, N. W. Navigating the microbiota seas: triangulation finds a way forward. Cell Host Microbe 23, 1–3 (2018).
Poesen, R. et al. The influence of CKD on colonic microbial metabolism. J. Am. Soc. Nephrol. 27, 1389–1399 (2016).
Mondot, S. & Lepage, P. The human gut microbiome and its dysfunctions through the meta-omics prism. Ann. NY Acad. Sci. 1372, 9–19 (2016).
Rosen, C. E. & Palm, N. W. Functional classification of the gut microbiota: the key to cracking the microbiota composition code: functional classifications of the gut microbiota reveal previously hidden contributions of indigenous gut bacteria to human health and disease. Bioessays 39, 1700032 (2017).
Wyatt, R. J. & Julian, B. A. IgA nephropathy. N. Engl. J. Med. 368, 2402–2414 (2013).
Barratt, J., Smith, A. C., Molyneux, K. & Feehally, J. Immunopathogenesis of IgAN. Semin. Immunopathol. 29, 427–443 (2007).
Kiryluk, K. et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat. Genet. 46, 1187–1196 (2014).
Fellstrom, B. C. et al. Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double-blind, randomised, placebo-controlled phase 2b trial. Lancet 389, 2117–2127 (2017).
Dong, J. Y. et al. Effect of probiotic fermented milk on blood pressure: a meta-analysis of randomised controlled trials. Br. J. Nutr. 110, 1188–1194 (2013).
Khalesi, S., Sun, J., Buys, N. & Jayasinghe, R. Effect of probiotics on blood pressure: a systematic review and meta-analysis of randomized, controlled trials. Hypertension 64, 897–903 (2014).
Hatch, M., Gjymishka, A., Salido, E. C., Allison, M. J. & Freel, R. W. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G461–G469 (2011).
Milliner, D., Hoppe, B. & Groothoff, J. A randomised Phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis 46, 313–323 (2018).
Goldfarb, D. S., Modersitzki, F. & Asplin, J. R. A randomized, controlled trial of lactic acid bacteria for idiopathic hyperoxaluria. Clin. J. Am. Soc. Nephrol. 2, 745–749 (2007).
Lieske, J. C. et al. Diet, but not oral probiotics, effectively reduces urinary oxalate excretion and calcium oxalate supersaturation. Kidney Int. 78, 1178–1185 (2010).
Acknowledgements
F.K. is supported by the Deutsche Forschungsgemeinschaft (DFG; project KN 1148/2-1 and CRC 1365 Renoprotection), the Oxalosis and Hyperoxaluria Foundation and TRENAL, a thematic network grant of the Deutscher Akademischer Austauschdienst (DAAD). J.R.B. is supported by an American Cancer Society Postdoctoral Fellowship (PF-17-237-01-DMC). R.A.F. is supported by the Howard Hughes Medical Institute.
Reviewer information
Nature Reviews Nephrology thanks C. M. Higueras, C. Kurts, L. Nazzal and other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
F.K. and J.R.B. researched data for the article and wrote the article. All authors contributed substantially to discussion of the article’s content and reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R.A.F. is a scientific consultant to GlaxoSmithKline and Zie Labs and a founder, shareholder and advisor to SMOC Therapeutics Inc. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Paneth cells
-
Epithelial cells localized in the glands of the small intestine. Microscopically, they are characterized by large eosinophilic granules that occupy the cytoplasm and contain antimicrobial compounds critical for host defence.
- Enterocytes
-
Epithelial cells found in the small intestine. Microscopically, they are characterized by microvilli found on the apical membrane. Enterocytes are responsible for the absorption or secretion of molecules from or into the intestine.
- Macrophage foam cells
-
Monocyte-derived macrophages that clear oxidized lipids and in this process develop into lipid-laden foam cells. These cells are components of atherosclerotic plaques and have been implicated in the pathogenesis of atherosclerotic disease.
- Coprophagic animals
-
Animals that eat faeces, which facilitates the transmission of microbiomes between individual animals.
- Goblet cells
-
Epithelial cells found in various organs. Goblet cells secrete mucus, a viscous fluid composed primarily of proteins called mucins.
- T helper 2 (TH2) cells
-
Specialized population of T cells that orchestrate protective type 2 immune responses to pathogens such as parasites as well as tissue repair. TH2 cells have also been implicated in diseases such as asthma.
- Pathogen-associated molecular patterns
-
(PAMPs). Highly conserved groups of molecular motifs detected by pattern recognition receptor (PRR)-bearing cells of the innate immune system.
- Natural killer T (NKT) cells
-
Subset of T cells that express both a T cell receptor, a classical component of adaptive immunity, and surface receptors for natural killer cells, which are characteristic of innate immunity.
- Immune exclusion
-
Process of clearing pathogenic microorganisms. Secretory immunoglobulin A (IgA) has a central role in this process by blocking antigens and pathogenic microorganisms from the intestinal lumen.
- Vivaria
-
Enclosed areas for live animals in a semi-natural environment for observation or studies.
- Histone deacetylases
-
(HDACs). Class of enzymes that remove acetyl groups. HDACs regulate access to DNA by modulating chromatin and thereby cellular processes. HDACs are also involved in immunity by, for example, regulating CD4+ T cells.
- Paracellular transport
-
Transfer of molecules or solutes across the epithelium by passing through the intercellular space between cells. This route of transport can be enhanced if junctional proteins are displaced or have altered expression.
- Transcellular transport
-
Transfer of molecules or solutes across the epithelium by passing through the cell. This route of transport is mediated by specialized proteins that transport from the lumen to the blood (absorption) or from the blood to the lumen (secretion).
- Metagenomics
-
Direct genetic analysis of entire microbial communities to provide information on microbial diversity.
- Metaproteomics
-
Characterization of all the protein samples recovered from environmental sources.
Rights and permissions
About this article
Cite this article
Knauf, F., Brewer, J.R. & Flavell, R.A. Immunity, microbiota and kidney disease. Nat Rev Nephrol 15, 263–274 (2019). https://doi.org/10.1038/s41581-019-0118-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-019-0118-7
This article is cited by
-
Causal effects of inflammatory bowel diseases on the risk of kidney stone disease: a two-sample bidirectional mendelian randomization
BMC Urology (2023)
-
Association between circadian syndrome and the prevalence of kidney stones in overweight adults: a cross-sectional analysis of NHANES 2007–2018
BMC Public Health (2023)
-
Washed microbiota transplantation improves renal function in patients with renal dysfunction: a retrospective cohort study
Journal of Translational Medicine (2023)
-
Hydrangea paniculata coumarins attenuate experimental membranous nephritis by bidirectional interactions with the gut microbiota
Communications Biology (2023)
-
Gut microbiota and neonatal acute kidney injury biomarkers
Pediatric Nephrology (2023)