Abstract
Purpose
The purpose of the study was to determine the screening performance of prenatal reflex DNA screening for trisomies 21 (T21), 18 (T18), and 13 (T13) as part of a routine service at five hospitals.
Methods
Women who accepted screening had a first-trimester combined test (pregnancy-associated plasma protein A, free β-human chorionic gonadotropin, nuchal translucency interpreted with maternal age). Those with a risk of having an affected pregnancy ≥1 in 800 were reflexed to a DNA sequencing test using stored plasma from the original blood sample, thereby avoiding the need to recall them.
Results
Of 22,812 women screened (including 106 with affected pregnancies), 2,480 (10.9%) were reflexed to DNA testing; 101/106 were detected (69/73 T21, 24/25 T18, and 8/8 T13), a 95% detection rate (95% confidence interval 89–98%) with four false positives (0.02%, 95% confidence interval 0.00–0.05%). The odds of being affected given a positive result were 25:1. Of the 105 screen-positive pregnancies, 91 (87%) had an invasive diagnostic test. Reflex DNA screening avoided up to 530 invasive diagnostic tests compared with using the combined test.
Conclusion
Reflex DNA screening was successfully implemented in routine care, achieving a high detection rate, low false-positive rate, and, consequently, greater safety with fewer invasive diagnostic tests than other methods of screening.
Similar content being viewed by others
Introduction
Prenatal screening for trisomy 21 (Down syndrome), trisomy 18 (Edwards syndrome), and trisomy 13 (Patau syndrome) using plasma (cell-free) DNA analysis offers substantial improvements over conventional screening methods, which are based on ultrasound and serum markers.1,2 However, DNA analysis is complex and relatively costly, and has a technical failure rate of a few percent, particularly when the percentage of cell-free DNA from the placenta is low. For these reasons DNA analysis has not generally been adopted as a method of primary screening. A two-step method has been proposed, in which the first-trimester combined test (based on the measurement of nuchal translucency, pregnancy-associated plasma protein A, free β-human chorionic gonadotropin, and maternal age) is followed by a DNA screening test (also known as a noninvasive prenatal test, NIPT) if the combined test risk estimate is above a specified level. Women are recalled for the DNA test. This approach (“contingent screening”) has been the subject of various studies.3,4,5,6 A weakness with this approach is the need to recall women for counseling and to obtain an extra blood sample for the DNA screening test. This recall is likely to make the women acutely anxious; some may choose to proceed directly to an amniocentesis or chorionic villus sampling with the associated risks to the pregnancy. To avoid causing this recall-induced anxiety, while still achieving a high screening performance, we proposed a modified screening method: reflex DNA screening.7,8 The method also avoids the clinical dilemma regarding the management of pregnancies with test failures. In reflex DNA screening an additional plasma sample is obtained at the time of blood collection for the combined test, and retained for potential DNA testing if the woman has a combined test risk of a trisomy 21, trisomy 18, or trisomy 13 pregnancy exceeding a prespecified cutoff. The DNA test is automatically triggered (i.e., a reflex response) without having to recall women for counseling to obtain an extra blood sample for the DNA test. In these women a combined test is not reported as a separate test but the information obtained from the combined test markers is used together with the information gained from the DNA test to calculate the overall risk, which is reported. The first-trimester combined test is thus never “positive.” If the combined test risk is below the prespecified cutoff level used to trigger a DNA analysis the combined test result is reported as negative. The screening protocol is shown in Figure 1. This protocol has the advantage that each woman has only one screening test, i.e., either a combined test, which is always reported as negative, or a DNA test that includes information from the combined test markers, which can be reported as positive or negative.
Here we describe the completed implementation project based on 22,812 unselected pregnancies screened between 1 April 2015 and 31 August 2016.
Materials and methods
Five hospitals participated in the prenatal reflex DNA screening implementation project: the Royal London, Whipps Cross, Newham, Kingston, and Liverpool Women’s. Women who agreed to have a screening test for trisomies 21, 18, and 13 (the three disorders referred to as affected pregnancies) received a leaflet describing the reflex DNA screening approach. Blood for the serum and plasma samples was collected in a plain tube and an anticoagulant tube (Streck, Omaha, NE, tubes formulated to stabilize blood cells) between 11 and 13 completed weeks of pregnancy (three pregnancies were tested in the 10th completed week of pregnancy). The combined test was performed on the serum sample using Roche (Basel, Switzerland) pregnancy-associated plasma protein A and free β-human chorionic gonadotropin assays. Combined test risks were calculated separately for trisomy 21, trisomy 18, and trisomy 13 using the αlpha software (Logical Medical Systems, London, UK). The software was validated against independent software produced at the Wolfson Institute of Preventive Medicine.
If the combined test risk for any of the three disorders was ≥1 in 800 (chosen from previous modeling to identify about 10% of unaffected pregnancies),7 the previously collected plasma sample was used for the DNA test using massively parallel shotgun sequencing. Up to 21 October 2015 this was carried out by Sequenom in San Diego, CA (715 pregnancies), using an Illumina (San Diego, CA) platform.9,10 Thereafter, the DNA tests were performed at the Wolfson Institute of Preventive Medicine (1,765 pregnancies) using an Ion Proton Thermo Fisher (Waltham, MA) platform supplied by Premaitha, Manchester, UK, with associated reagents, chips, and assay software.11
The plasma sample was usually sufficient for a second DNA analysis if the first failed. If both analyses failed, women were invited to attend again to provide another blood sample. This was again divided into two portions, one plasma and one serum, the plasma sample for a DNA test, and the serum sample to measure second-trimester quadruple test markers—alpha-fetoprotein, human chorionic gonadotropin (Roche), unconjugated estriol, and inhibin A (Beckman Coulter, Brea, CA)—if the DNA failed. These were used together with the first-trimester markers as an integrated test. A risk ≥1 in 150 was considered a positive result.
Results from Sequenom and the Wolfson Institute DNA analyses were reported as the risks of having a pregnancy with trisomies 21, 18, and 13. From 22 October 2015 two changes were made: (i) the risks reported were refined by combining the DNA likelihood ratios with the combined test risk, and (ii) a reported risk cutoff of 1 in 150 was used (standard in the United Kingdom) instead of 1 in 100 as used by Sequenom.
Data on whether the pregnancy was affected by trisomy 21, 18, or 13 were obtained from the prenatal screening coordinator at each participating hospital. Among pregnancies with positive DNA results the following were excluded from our estimates of screening performance: (i) 12 pregnancies that ended in a miscarriage or intrauterine death (two trisomy 21, three trisomy 18, seven without a karyotype); (ii) 3 pregnancies that had a termination of pregnancy without karyotype, a decision influenced by findings seen on the ultrasound scan; and (iii) 1 pregnancy that was lost to follow-up. In addition, seven women did not complete the screening protocol because they declined to return for an extra blood collection, four of whom chose to have an amniocentesis directly (three trisomy 21, one unaffected). The detection rate was defined as the proportion of affected pregnancies with a positive screening result. The false-positive rate was defined as the proportion of unaffected pregnancies with a positive screening result. Research ethics committee approval was not required as the project was an audit of the implementation of prenatal reflex DNA screening as a clinical service.
Results
Table 1 shows selected characteristics of the 22,812 pregnancies screened among women who completed the screening protocol and for whom there was information on whether the pregnancies were affected or unaffected. There were 73 trisomy 21, 25 trisomy 18, and 8 trisomy 13 pregnancies (combined trisomy rate 0.46%).
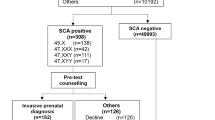
Figure 2 is a flow diagram showing the results of the reflex DNA screening program according to whether the pregnancies were affected or unaffected. The figure shows that 10.5% of unaffected pregnancies and 97% of affected pregnancies had a combined test risk ≥1 in 800 and consequently were reflexed to the DNA test. Following the DNA test, 101 affected pregnancies (69 trisomy 21, 24 trisomy 18, and 8 trisomy 13) were screen positive, yielding a detection rate of 95% (95% confidence interval, 89–98%, see Table 2). Four unaffected pregnancies were screen positive, yielding a false-positive rate of 0.02% (95% confidence interval, 0.00–0.05%, see Table 2). The odds of being affected given a positive result from the screening program were 25:1 (positive predictive value of 96.2% (25/26)). Of the 101 women with affected pregnancies who were screen positive, 88 (87%) had an invasive diagnostic test, and of these 83 (94%) had a termination. Of the four women with a false-positive result, three had an invasive diagnostic test. Included in the overall results were data on 381 twin pregnancies; 39 were reflexed to a DNA test, 4 had positive DNA results, and all were affected with trisomy 21. The median duration from blood sampling to informing a woman of a positive screening result was 11 days (interquartile range 9 to 13). Negative results were mailed within 2 weeks.
Table 2 shows the observed performance of reflex DNA screening compared with screening using the combined test only with a risk cutoff of 1 in 150, as is standard in the United Kingdom. Reflex DNA screening had a detection rate of 95%, 14 percentage points higher than screening using the combined test alone, a 100-fold reduction in the false-positive rate (0.02% vs. 2.42%), and an odds of being affected given a positive result 150 times higher (25:1/1:6 = 150).
Supplementary Table 1 online shows the proportion of pregnancies that would have been reflexed to a DNA test according to the combined test risk cutoff level. As the cutoff level is increased, the proportion of pregnancies reflexed to a DNA test decreases, for example from 10.9% using a combined test cutoff 1 of in 800 (97% for affected pregnancies and 10.5% for unaffected (see Supplementary Table 1)) to 2.8% (81% of affected pregnancies and 2.4% of unaffected pregnancies) using a 1 in 150 cutoff.
Supplementary Table 2 shows the screening performance using different reflex combined test risk cutoff levels. The performance depends on the combined test risk cutoff used. If DNA testing had been restricted to women with a combined test risk of ≥1 in 150, the detection rate would be 79%, a 16–percentage point reduction compared with using a 1 in 800 cutoff. The false positive rate is, however, unchanged.
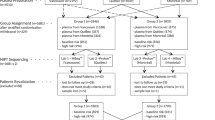
Figure 3 shows the DNA technical failure rates, indicating failures arising in the first and second aliquots of the initial blood collection and the first and second aliquots of an extra blood collection. This analysis was limited to the 1,756 pregnancies tested at the Wolfson Institute, where the necessary records on test failure and retesting were maintained. In 8.7% (152/1,756) of pregnancies there was a test failure using the first aliquot. An extra blood collection was required in 1.8% of pregnancies (1.7% + 0.1% in Figure 3). There were no reported adverse outcomes in pregnancies in which DNA testing failed. There was a suggestion that maternal weight was higher in women who had a repeat blood collection (median 69 vs. 65 kg), but this was not statistically significant (P = 0.055). Obtaining a second blood sample for a repeat DNA test was not more common earlier in pregnancy than later.
Discussion
This implementation project showed that routine reflex DNA screening for trisomies 21, 18, and 13 achieved a detection rate of 95% with a false-positive rate of 0.02% and an odds of being affected given a positive result of 25:1. Only 2 in 10,000 women with unaffected pregnancies had an invasive diagnostic test. No other method of prenatal screening for these disorders has such a high detection rate for such a low false-positive rate. While the detection rate is a few percentage points lower than with universal DNA screening,9,10,12 the greater proportional reduction in the false-positive rate results in a greater discrimination between affected and unaffected pregnancies.
Chitty et al.3 described a similar two-step screening protocol but, instead of performing a reflex DNA test on a previously collected plasma sample, women with a combined test risk ≥1 in 1,000 were recalled for counseling with the offer of a DNA screening test or, if the risk was ≥1 in 150, the choice of a DNA screening test or an invasive diagnostic test. Twelve percent of women were recalled in this way and informed that they were in this higher risk group, and 18% chose to proceed directly to an invasive diagnostic test. This increases the false-positive rate and consequently also increases the number of invasive diagnostic tests in women with unaffected pregnancies; this is avoided with the reflex method. We can be confident that the reflex DNA screening strategy benefits women by reducing the chance that they will be made acutely anxious. Measuring anxiety levels directly in such circumstances is, in our view, neither appropriate nor necessary; imparting potentially distressing information when this can be completely avoided is self-evidently of benefit.
The technical DNA test failure rate is a problem with DNA screening. The reflex DNA approach with 10% of women having a DNA test means that, among all women screened, about 2 per 1,000 (10% × 1.8%) needed to have an extra blood collection, a much lower recall rate than with contingent DNA screening without reflexing.3 The 68% reduction in the failure rate between tests using the first and second aliquot from the initial blood sample indicates that the failure is mainly technical, and not due to factors associated with the woman and her pregnancy. As the technical aspects of the test improve, the initial failure rate is likely to fall significantly. With the reflex DNA screening approach, all pregnancies have a screening result and in this implementation project only 3 pregnancies out of 2,480 reflexed to a DNA test (0.12%) had an integrated test after a DNA test failure using the second aliquot of the extra blood collection.
Compared with established screening methods, reflex DNA screening reduces the clinical workload involved in counseling women with screen-positive results; in the implementation audit 105 (101 + 4 from Table 2) women required counseling following a screen-positive result whereas 635 (549 + 86) would have required counseling if the combined test alone or the two-step (recall) method 3 had been used. The two-step method 3 will further increase the clinical workload because in addition to counseling after a positive combined test result, some women would need counseling again after a positive DNA test result.
Women identified as being screen positive in the implementation audit had a high odds of having an affected pregnancy (25:1, see Table 2), which is likely to reduce uncertainty over the decision to have an invasive diagnostic test (1:6 with the combined test alone, see Table 2). Reflex DNA screening can potentially achieve cost savings because of the reduction in the number of invasive diagnostic tests needed and the reduced need for patient counseling associated with the two-step approach. These savings could be used to pay for the reflex DNA screening tests to secure the clinical benefits. Depending on local costs, a combined test cutoff level could be selected so that the costs of the screening program are affordable and cost-effective. As the cost of reflex DNA screening declines, the combined test cutoff can be lowered, resulting in an increase in the proportion of women having a reflex DNA test and hence an increase in the detection rate.
In this implementation project Streck tubes were used to reduce white blood cell lysis and cellular DNA leakage into the plasma of whole blood before plasma separation. The effect of such leakage would be to diminish the fetal fraction and possibly increase the proportion of failed tests. The use of inexpensive ethylenediaminetetraacetic acid (EDTA) tubes rather than the more expensive Streck tubes would lead to cost savings. Such a switch should be acceptable as there is evidence that the separation of plasma from cells up to at least 48 hours after blood collection does not significantly degrade the sample needed for DNA analysis.13,14
Integrating information from combined test markers with sequencing information from DNA analysis enhances screening performance. While the improvement in screening performance is small, with suitable interpretive software this can readily be implemented without additional cost. A source of false positives associated with the DNA test arises from maternal mosaicism,15 confined placental mosaicism,16 and maternal copy-number variation.17 Though they are rare occurrences, this problem is mitigated in reflex DNA screening. For example, if 10% of women have a reflex DNA test the problem is reduced 10-fold. A practical point affecting any reflex DNA screening program is that the invasive diagnostic test should be amniocentesis, not chorionic villus sampling, which will replicate the confined placental mosaicism observed in the maternal plasma.
With universal DNA screening the detection rate would have been 99%, but an extra blood sample would be required for a repeat DNA test in 1.8% of pregnancies based on the failure rate in pregnancies tested at the Wolfson Institute (see Figure 3). With the reflex approach, in which 10% have a DNA test, the detection rate is 95% with a 10-fold lower rate of requesting an extra blood collection (0.18%) than universal DNA screening. There is a trade-off between small incremental increases in detection for increasing proportions of women required to provide an extra blood sample. In every 100,000 pregnancies undergoing reflex DNA screening, based on a 10% reflexing proportion, 180 would be recalled for an extra blood collection compared with 1,800 such return visits with universal DNA screening.
The reflex DNA policy makes prenatal screening for trisomies 21, 18, and 13 safer than other policies because of the reduced false-positive rate. Taking the risk of fetal loss due to an invasive diagnostic test as 1 in 100,18 among 1 million unaffected pregnancies that undergo reflex DNA screening, 200 would have a diagnostic amniocentesis and about 2 of these would result in a fetal loss due to the diagnostic procedure. If all pregnant women were screened using the reflex DNA approach, this would amount to eight procedure-related unaffected fetal losses in the United States and about two in the United Kingdom, each year. If the fetal loss rate from an invasive diagnostic test is less than 1 in 100, these estimates of the number of procedure-related fetal losses would be even lower. As well as improved safety, 19 out of 20 pregnancies with trisomy 21, 18, or 13 are detected by reflex DNA screening.
The benefits of reflex DNA screening arise mainly from the substantially lower false-positive rate compared with other methods of screening, the avoidance of recall-induced anxiety associated with non-reflex contingent screening, and a detection rate similar to universal DNA testing. These clinical benefits, together with the reduced cost compared with universal DNA testing, make the reflex approach a preferred method of screening. The results of this implementation project show that the benefits of reflex DNA screening were achieved in routine screening practice.
References
Gregg AR, Skotko BG, Benkendorf JL et al. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med 2016;18:1056–65.
American College of Obstetricians and Gynecologists. Committee opinion no. 640: cell free DN screening for fetal aneuploidy. Obstet Gynecol 2015;126:e31–7.
Chitty LS, Wright D, Hill M et al. Uptake, outcomes, and costs of implementing non-invasive prenatal testing for Down’s syndrome into NHS maternity care: prospective cohort study in eight diverse maternity units. BMJ 2016;354:i3426.
Oepkes D, Page-Christiaens GC, Bax CJ et al. Trial by Dutch laboratories for evaluation of non-invasive prenatal testing. Part 1—clinical impact. Prenat Diagn 2016;26:1083–90.
Huang T, Meschino WS, Teitelbaum M, Dougan S, Okun N. Enhanced first trimester screening for Trisomy 21 with contingent cell-free fetal DNA: a comparative performance and cost analysis. J Obstet Gynaecol Can 2017;39:742–749.
Miltoft CB, Rode L, Ekelund CK et al. Contingent first-trimester screening for aneuploidies with cell-free DNA in a Danish clinical setting. Ultrasound Obstet Gynecol; e-pub ahead of print 22 June 2017.
Wald NJ, Bestwick JP. Performance of antenatal reflex DNA screening for Down’s syndrome. J Med Screen 2015;22:168–174.
Wald NJ, Huttly WJ, Bestwick JP, Aquilina J, Peregrine E. Reflex antenatal DNA screening for Down syndrome. Prenat Diagn 2015;35:1154.
Palomaki GE, Kloza EM, Lambert-Messerlian GM et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study. Genet Med 2011;13:913–20.
Palomaki GE, Deciu C, Kloza EM et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: an international collaborative study. Genet Med 2012;14:296–30.
Crea F, Forman M, Hulme R et al. The IONA test: development of an automated cell-free DNA-based screening test for fetal trisomies 13, 18, and 21 that employs the ion proton semiconductor sequencing platform. Fetal Diagn Ther 2017;42:218–224.
Gil MM, Accurti V, Santacruz B, Plana MN, Nicolaides KH. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol 2017;50:302–314.
Buysse K, Beulen L, Gomes I et al. Reliable noninvasive prenatal testing by massively parallel sequencing of circulating cell-free DNA from maternal plasma processed up to 24h after venipuncture. Clin Biochem 2013;46:1783–6.
Diaz EH, Yachnin J, Grönberg H, Lindberg J. The in vitro stability of circulating tumour DNA. PLoS One 2016;11:e0166153.
Lau TK, Jiang FM, Stevenson RJ et al. Secondary findings from non-invasive prenatal testing for common fetal aneuploidies by whole genome sequencing as a clinical service. Prenat Diagn 2013;33:602–8.
Grati FR, Malvestiti F, Ferreira JCPB et al. Fetoplacental mosaicism: potential implications for false-positive and false-negative noninvasive prenatal screening results. Genet Med 2014;16:620–4.
Snyder MW, LaVone MS, Simmons E et al. Copy-number variation and false positive prenatal aneuploidy screening results. N Engl J Med 2015;372:1639–45.
Gosden C, Tabor A, Leck I, Grant A, Alfirevic Z, Wald NAmniocentesis and chorionic villus sampling. In: Antenatal and Neonatal ScreeningWald NJ, Leck I (eds). Oxford University Press: Oxford, UK,, 2000470–516.
Acknowledgments
We thank the screening coordinators at the participating hospitals: Sherry-Ann Francis (Royal London), Deborah Jordan (Newham), Jenise Jarvis (Newham coordinator when project started), Folakemi Idialu (Whipps Cross), Heather Longworth (Liverpool Women’s), and Balvinder Reehal (Kingston).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
N.J.W. is director of Logical Medical Systems, which produces software for the interpretation of prenatal screening tests. The other authors declare no conflict of interest.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Wald, N.J., Huttly, W.J., Bestwick, J.P. et al. Prenatal reflex DNA screening for trisomies 21, 18, and 13. Genet Med 20, 825–830 (2018). https://doi.org/10.1038/gim.2017.188
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/gim.2017.188
Keywords
This article is cited by
-
A new contingent screening strategy increased detection rate of trisomy 21 in the first trimester
BMC Pregnancy and Childbirth (2023)
-
Nanostructures in non-invasive prenatal genetic screening
Biomedical Engineering Letters (2022)
-
Prenatal screening for trisomy 21: a comparative performance and cost analysis of different screening strategies
BMC Pregnancy and Childbirth (2020)
-
A Retrospective Analysis Of Different Contingent Screening Models For Fetal Down Syndrome In Southwestern China
Scientific Reports (2020)
-
Response to Wald et al
Genetics in Medicine (2018)