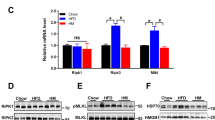
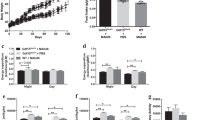
Abstract
Adult neural stem cells (NSCs) are known to exist in a few regions of the brain; however, the entity and physiological/disease relevance of adult hypothalamic NSCs (htNSCs) remain unclear. This work shows that adult htNSCs are multipotent and predominantly present in the mediobasal hypothalamus of adult mice. Chronic high-fat-diet feeding led to not only depletion but also neurogenic impairment of htNSCs associated with IKKβ/NF-κB activation. In vitro htNSC models demonstrated that their survival and neurogenesis markedly decreased on IKKβ/NF-κB activation but increased on IKKβ/NF-κB inhibition, mechanistically mediated by IKKβ/NF-κB-controlled apoptosis and Notch signalling. Mouse studies revealed that htNSC-specific IKKβ/NF-κB activation led to depletion and impaired neuronal differentiation of htNSCs, and ultimately the development of obesity and pre-diabetes. In conclusion, adult htNSCs are important for the central regulation of metabolic physiology, and IKKβ/NF-κB-mediated impairment of adult htNSCs is a critical neurodegenerative mechanism for obesity and related diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Reynolds, B. A. & Weiss, S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707–1710 (1992).
Ray, J., Peterson, D. A., Schinstine, M. & Gage, F. H. Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc. Natl Acad. Sci. USA 90, 3602–3606 (1993).
Cameron, H. A. & McKay, R. Stem cells and neurogenesis in the adult brain. Curr. Opin. Neurobiol. 8, 677–680 (1998).
Johansson, C. B. et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96, 25–34 (1999).
Gage, F. H. Mammalian neural stem cells. Science 287, 1433–1438 (2000).
Gross, C. G. Neurogenesis in the adult brain: death of a dogma. Nat. Rev. Neurosci. 1, 67–73 (2000).
Morrison, S. J. Neuronal potential and lineage determination by neural stem cells. Curr. Opin. Cell Biol. 13, 666–672 (2001).
Varez-Buylla, A. & Lim, D. A. For the long run: maintaining germinal niches in the adult brain. Neuron 41, 683–686 (2004).
Emsley, J. G., Mitchell, B. D., Kempermann, G. & Macklis, J. D. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog. Neurobiol. 75, 321–341 (2005).
Gould, E. How widespread is adult neurogenesis in mammals? Nat. Rev. Neurosci. 8, 481–488 (2007).
Whitman, M. C. & Greer, C. A. Adult neurogenesis and the olfactory system. Prog. Neurobiol. 89, 162–175 (2009).
Temple, S. The development of neural stem cells. Nature 414, 112–117 (2001).
Doetsch, F., Caille, I., Lim, D. A., Garcia-Verdugo, J. M. & varez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703–716 (1999).
Merkle, F. T., Mirzadeh, Z. & varez-Buylla, A. Mosaic organization of neural stem cells in the adult brain. Science 317, 381–384 (2007).
Kriegstein, A. & varez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184 (2009).
Kokoeva, M. V., Yin, H. & Flier, J. S. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science 310, 679–683 (2005).
Kokoeva, M. V., Yin, H. & Flier, J. S. Evidence for constitutive neural cell proliferation in the adult murine hypothalamus. J. Comput. Neurol. 505, 209–220 (2007).
Pierce, A. A. & Xu, A. W. De novo neurogenesis in adult hypothalamus as a compensatory mechanism to regulate energy balance. J. Neurosci. 30, 723–730 (2010).
McNay, D. E., Briancon, N., Kokoeva, M. V., Maratos-Flier, E. & Flier, J. S. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J. Clin. Invest 122, 142–152 (2012).
Lee, D. A. et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 15, 700–702 (2012).
Purkayastha, S. et al. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc. Natl Acad. Sci. USA 108, 2939–2944 (2011).
Zhang, X. et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Cai, D. & Liu, T. Hypothalamic inflammation: A double-edged sword to nutritional diseases. Ann. N. Y. Acad. Sci. 1243, E1–E39 (2011).
Cai, D. & Liu, T. Inflammatory cause of metabolic syndrome via brain stress and NF-κB. Aging 4, 98–115 (2012).
Purkayastha, S., Zhang, G. & Cai, D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat. Med. 17, 883–887 (2011).
Hayden, M. S., West, A. P. & Ghosh, S. SnapShot: NF-κB signaling pathways. Cell 127, 1286–1287 (2006).
Hoffmann, A. & Baltimore, D. Circuitry of nuclear factor κB signaling. Immunol. Rev. 210, 171–186 (2006).
Li, Q. & Verma, I. M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2, 725–734 (2002).
Karin, M. & Lin, A. NF-κB at the crossroads of life and death. Nat. Immunol. 3, 221–227 (2002).
Vousden, K. H. Partners in death: a role for p73 and NF-kB in promoting apoptosis. Aging 1, 275–277 (2009).
Dutta, J., Fan, Y., Gupta, N., Fan, G. & Gelinas, C. Current insights into the regulation of programmed cell death by NF-κB. Oncogene 25, 6800–6816 (2006).
Rolls, A. et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 9, 1081–1088 (2007).
Koo, J. W. & Duman, R. S. IL-1β is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl Acad. Sci. USA 105, 751–756 (2008).
Koo, J. W., Russo, S. J., Ferguson, D., Nestler, E. J. & Duman, R. S. Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl Acad. Sci. USA 107, 2669–2674 (2010).
Is-Donini, S. et al. Impaired adult neurogenesis associated with short-term memory defects in NF-κB p50-deficient mice. J. Neurosci. 28, 3911–3919 (2008).
Martino, G. & Pluchino, S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 7, 395–406 (2006).
Villeda, S. & Wyss-Coray, T. Microglia–a wrench in the running wheel? Neuron 59, 527–529 (2008).
Villeda, S. A. et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90–94 (2011).
Niswender, K. D., Baskin, D. G. & Schwartz, M. W. Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol. Metab 15, 362–369 (2004).
Munzberg, H. & Myers, M. G. Jr Molecular and anatomical determinants of central leptin resistance. Nat. Neurosci. 8, 566–570 (2005).
Flier, J. S. Neuroscience. Regulating energy balance: the substrate strikes back. Science 312, 861–864 (2006).
Coll, A. P., Farooqi, I. S. & O’Rahilly, S. The hormonal control of food intake. Cell 129, 251–262 (2007).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest 122, 153–162 (2012).
Suh, H. et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1, 515–528 (2007).
Gilyarov, A. V. Nestin in central nervous system cells. Neurosci. Behav. Physiol 38, 165–169 (2008).
Vacca, A. et al. Notch3 and pre-TCR interaction unveils distinct NF-κB pathways in T-cell development and leukemia. EMBO J. 25, 1000–1008 (2006).
Oakley, F. et al. Basal expression of IκBα is controlled by the mammalian transcriptional repressor RBP-J (CBF1) and its activator Notch1. J. Biol. Chem. 278, 24359–24370 (2003).
Espinosa, L., Ingles-Esteve, J., Robert-Moreno, A. & Bigas, A. IκBα and p65 regulate the cytoplasmic shuttling of nuclear corepressors: cross-talk between Notch and NFκB pathways. Mol. Biol. Cell 14, 491–502 (2003).
Cheng, P. et al. Notch-1 regulates NF-κB activity in hemopoietic progenitor cells. J. Immunol. 167, 4458–4467 (2001).
Borghese, L. et al. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells 28, 955–964 (2010).
Carlen, M. et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci. 12, 259–267 (2009).
Louvi, A. & Artavanis-Tsakonas, S. Notch signalling in vertebrate neural development. Nat. Rev. Neurosci. 7, 93–102 (2006).
Oya, S. et al. Attenuation of Notch signaling promotes the differentiation of neural progenitors into neurons in the hippocampal CA1 region after ischemic injury. Neuroscience 158, 683–692 (2009).
Lutolf, S., Radtke, F., Aguet, M., Suter, U. & Taylor, V. Notch1 is required forneuronal and glial differentiation in the cerebellum. Development 129, 373–385 (2002).
rtavanis-Tsakonas, S., Rand, M. D. & Lake, R. J. Notch signaling: cell fate control and signal integration in development. Science 284, 770–776 (1999).
Hansen, D. V., Lui, J. H., Parker, P. R. & Kriegstein, A. R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561 (2010).
Zhang, G. et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron 69, 523–535 (2011).
Acknowledgements
The authors sincerely thank F. H. Gage (The Salk Institute for Biological Studies, USA) for the Sox2-promoter-containing lentiviral vector, D. Hickstein (National Cancer Institute, National Institutes of Health, USA) for the CD11b-promoter cDNA, and the members of the Cai laboratory for general technical assistance. This study was supported by Albert Einstein College of Medicine internal start-up funds and NIH R01 DK078750 and R01 AG031774 (all to D. Cai).
Author information
Authors and Affiliations
Contributions
D.C. conceived the project and designed the study. J.L. carried out all experiments and participated in the experimental design. Y.T. generated luciferase constructs of wild-type and mutant Notch signalling element gene promoters. J.L. and D.C. carried out data interpretation and discussion. D.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1914 kb)
Supplementary Table 1
Supplementary Information (XLS 30 kb)
Rights and permissions
About this article
Cite this article
Li, J., Tang, Y. & Cai, D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol 14, 999–1012 (2012). https://doi.org/10.1038/ncb2562
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2562
This article is cited by
-
Dysfunction of the hypothalamic-pituitary adrenal axis and its influence on aging: the role of the hypothalamus
Scientific Reports (2023)
-
An analogue of the Prolactin Releasing Peptide reduces obesity and promotes adult neurogenesis
EMBO Reports (2023)
-
Is metformin neuroprotective against diabetes mellitus-induced neurodegeneration? An updated graphical review of molecular basis
Pharmacological Reports (2023)
-
Glial cells as integrators of peripheral and central signals in the regulation of energy homeostasis
Nature Metabolism (2022)
-
Endoplasmic reticulum stress contributes to the decline in doublecortin expression in the immature neurons of mice with long-term obesity
Scientific Reports (2022)