Key Points
-
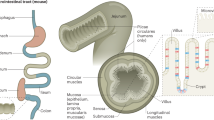
The brush border is a complex and highly plastic organelle required for intestinal homeostasis and is specialized for absorption of nutrients
-
Thousands of tightly packed microvilli form the brush border together with the area they are located on, the so-called terminal web
-
The brush border constitutes a biochemical and mechanical interface between the intestinal lumen and the internal milieu
-
Inherited or acquired genetic brush border defects lead to intestinal insufficiency and diarrhoea
-
Pathogens and inflammatory processes can alter the integrity of the brush border, either by actively remodelling or damaging its structure
Abstract
The brush border on the apical surface of enterocytes is a highly specialized structure well-adapted for efficient digestion and nutrient transport, whilst at the same time providing a protective barrier for the intestinal mucosa. The brush border is constituted of a densely ordered array of microvilli, protrusions of the plasma membrane, which are supported by actin-based microfilaments and interacting proteins and anchored in an apical network of actomyosin and intermediate filaments, the so-called terminal web. The highly dynamic, specialized apical domain is both an essential partner for the gut microbiota and an efficient signalling platform that enables adaptation to physiological stimuli from the external and internal milieu. Nevertheless, genetic alterations or various pathological stresses, such as infection, inflammation, and mechanical or nutritional alterations, can jeopardize this equilibrium and compromise intestinal functions. Long-time neglected, the intestinal brush-border shall be enlightening again as the central actor of the complex but essential intestinal homeostasis. Here, we review the processes and components involved in brush border organization and discuss pathological mechanisms that can induce brush border defects and their physiological consequences.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Revenu, C., Athman, R., Robine, S. & Louvard, D. The co-workers of actin filaments: from cell structures to signals. Nat. Rev. Mol. Cell Biol. 5, 635–646 (2004).
Crawley, S. W., Mooseker, M. S. & Tyska, M. J. Shaping the intestinal brush border. J. Cell Biol. 207, 441–451 (2014).
Tilney, L. G., Tilney, M. S. & DeRosier, D. J. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu. Rev. Cell Biol. 8, 257–274 (1992).
Maroux, S., Coudrier, E., Feracci, H., Gorvel, J. P. & Louvard, D. Molecular organization of the intestinal brush border. Biochimie 70, 1297–1306 (1988).
Louvard, D., Kedinger, M. & Hauri, H. P. The differentiating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu. Rev. Cell Biol. 8, 157–195 (1992).
Shifrin, D. A. Jr & Tyska, M. J. Ready...aim...fire into the lumen: a new role for enterocyte microvilli in gut host defense. Gut Microbes 3, 460–462 (2012).
Pollard, T. D. & Mooseker, M. S. Direct measurement of actin polymerization rate constants by electron microscopy of actin filaments nucleated by isolated microvillus cores. J. Cell Biol. 88, 654–659 (1981).
Mooseker, M. S. & Tilney, L. G. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J. Cell Biol. 67, 725–743 (1975).
Pollard, T. D. & Borisy, G. G. Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003).
Edwards, M. et al. Capping protein regulators fine-tune actin assembly dynamics. Nat. Rev. Mol. Cell Biol. 15, 677–689 (2014).
Bartles, J. R. Parallel actin bundles and their multiple actin-bundling proteins. Curr. Opin. Cell Biol. 12, 72–78 (2000).
Fehon, R. G., McClatchey, A. I. & Bretscher, A. Organizing the cell cortex: the role of ERM proteins. Nat. Rev. Mol. Cell Biol. 11, 276–287 (2010).
Cooper, J. A. & Pollard, T. D. Effect of capping protein on the kinetics of actin polymerization. Biochemistry 24, 793–799 (1985).
Caldwell, J. E., Heiss, S. G., Mermall, V. & Cooper, J. A. Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry 28, 8506–8514 (1989).
Schafer, D. A., Mooseker, M. S. & Cooper, J. A. Localization of capping protein in chicken epithelial cells by immunofluorescence and biochemical fractionation. J. Cell Biol. 118, 335–346 (1992).
Coluccio, L. M. & Bretscher, A. Reassociation of microvillar core proteins: making a microvillar core in vitro. J. Cell Biol. 108, 495–502 (1989).
Glenney, J. R. Jr, Bretscher, A. & Weber, K. Calcium control of the intestinal microvillus cytoskeleton: its implications for the regulation of microfilament organizations. Proc. Natl Acad. Sci. USA 77, 6458–6462 (1980).
Bretscher, A. & Weber, K. Fimbrin, a new microfilament-associated protein present in microvilli and other cell surface structures. J. Cell Biol. 86, 335–340 (1980).
Bretscher, A. & Weber, K. Villin: the major microfilament-associated protein of the intestinal microvillus. Proc. Natl Acad. Sci. USA 76, 2321–2325 (1979).
Bretscher, A. & Weber, K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell 20, 839–847 (1980).
Robine, S. et al. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? Proc. Natl Acad. Sci. USA 82, 8488–8492 (1985).
Costa de Beauregard, M. A., Pringault, E., Robine, S. & Louvard, D. Suppression of villin expression by antisense RNA impairs brush border assembly in polarized epithelial intestinal cells. EMBO J. 14, 409–421 (1995).
Pinson, K. I., Dunbar, L., Samuelson, L. & Gumucio, D. L. Targeted disruption of the mouse villin gene does not impair the morphogenesis of microvilli. Dev. Dyn. 211, 109–121 (1998).
George, S. P., Wang, Y., Mathew, S. Srinivasan, K. & Khurana, S. Dimerization and actin-bundling properties of villin and its role in the assembly of epithelial cell brush borders. J. Biol. Chem. 282, 26528–26541 (2007).
Friederich, E., Vancompernolle, K., Louvard, D. & Vandekerckhove, J. Villin function in the organization of the actin cytoskeleton. Correlation of in vivo effects to its biochemical activities in vitro. J. Biol. Chem. 274, 26751–26760 (1999).
Khurana, S. & George, S. P. Regulation of cell structure and function by actin-binding proteins: villin's perspective. FEBS Lett. 582, 2128–2139 (2008).
Kumar, N., Tomar, A., Parrill, A. L. & Khurana, S. Functional dissection and molecular characterization of calcium-sensitive actin-capping and actin-depolymerizing sites in villin. J. Biol. Chem. 279, 45036–45046 (2004).
Glenney, J. R. Jr, Geisler, N., Kaulfus, P. & Weber, K. Demonstration of at least two different actin-binding sites in villin, a calcium-regulated modulator of F-actin organization. J. Biol. Chem. 256, 8156–8161 (1981).
Ferrary, E. et al. In vivo, villin is required for Ca2+-dependent F-actin disruption in intestinal brush borders. J. Cell Biol. 146, 819–830 (1999).
Kumar, N. & Khurana, S. Identification of a functional switch for actin severing by cytoskeletal proteins. J. Biol. Chem. 279, 24915–24918 (2004).
Kumar, N., Zhao, P., Tomar, A., Galea, C. A. & Khurana, S. Association of villin with phosphatidylinositol 4,5-bisphosphate regulates the actin cytoskeleton. J. Biol. Chem. 279, 3096–3110 (2004).
Lin, C. S., Shen, W., Chen, Z. P., Tu, Y. H. & Matsudaira, P. Identification of I-plastin, a human fimbrin isoform expressed in intestine and kidney. Mol. Cell. Biol. 14, 2457–2467 (1994).
Grimm-Gunter, E. M. et al. Plastin 1 binds to keratin and is required for terminal web assembly in the intestinal epithelium. Mol. Biol. Cell 20, 2549–2562 (2009).
Bartles, J. R., Zheng, L., Li, A., Wierda, A. & Chen, B. Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J. Cell Biol. 143, 107–119 (1998).
Disanza, A. et al. Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8–IRSp53 complex. Nat. Cell Biol. 8, 1337–1347 (2006).
Hertzog, M. et al. Molecular basis for the dual function of Eps8 on actin dynamics: bundling and capping. PLoS Biol. 8, e1000387 (2010).
Croce, A. et al. A novel actin barbed-end-capping activity in EPS-8 regulates apical morphogenesis in intestinal cells of Caenorhabditis elegans. Nat. Cell Biol. 6, 1173–1179 (2004).
Berryman, M., Franck, Z. & Bretscher, A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J. Cell Sci. 105, 1025–1043 (1993).
Tyska, M. J. et al. Myosin-1a is critical for normal brush border structure and composition. Mol. Biol. Cell 16, 2443–2457 (2005).
Saotome, I., Curto, M. & McClatchey, A. I. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev. Cell 6, 855–864 (2004).
Fievet, B. T. et al. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J. Cell Biol. 164, 653–659 (2004).
Bretscher, A., Reczek, D. & Berryman, M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J. Cell Sci. 110, 3011–3018 (1997).
ten Klooster, J. P. et al. Mst4 and Ezrin induce brush borders downstream of the Lkb1/Strad/Mo25 polarization complex. Dev. Cell 16, 551–562 (2009).
Shiue, H., Musch, M. W., Wang, Y., Chang, E. B. & Turner, J. R. Akt2 phosphorylates ezrin to trigger NHE3 translocation and activation. J. Biol. Chem. 280, 1688–1695 (2005).
Wald, F. A. et al. Atypical protein kinase C (iota) activates ezrin in the apical domain of intestinal epithelial cells. J. Cell Sci. 121, 644–654 (2008).
Viswanatha, R., Ohouo, P. Y., Smolka, M. B. & Bretscher, A. Local phosphocycling mediated by LOK/SLK restricts ezrin function to the apical aspect of epithelial cells. J. Cell Biol. 199, 969–984 (2012).
Dhekne, H. S. et al. Myosin Vb and Rab11a regulate phosphorylation of ezrin in enterocytes. J. Cell Sci. 127, 1007–1017 (2014).
Reczek, D., Berryman, M. & Bretscher, A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J. Cell Biol. 139, 169–179 (1997).
Morales, F. C., Takahashi, Y., Kreimann, E. L. & Georgescu, M. M. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc. Natl Acad. Sci. USA 101, 17705–17710 (2004).
Garbett, D., LaLonde, D. P. & Bretscher, A. The scaffolding protein EBP50 regulates microvillar assembly in a phosphorylation-dependent manner. J. Cell Biol. 191, 397–413 (2010).
Chiba, H. et al. The nuclear receptor hepatocyte nuclear factor 4α acts as a morphogen to induce the formation of microvilli. J. Cell Biol. 175, 971–980 (2006).
LaLonde, D. P., Garbett, D. & Bretscher, A. A regulated complex of the scaffolding proteins PDZK1 and EBP50 with ezrin contribute to microvillar organization. Mol. Biol. Cell 21, 1519–1529 (2010).
Casaletto, J. B., Saotome, I., Curto, M. & McClatchey, A. I. Ezrin-mediated apical integrity is required for intestinal homeostasis. Proc. Natl Acad. Sci. USA 108, 11924–11929 (2011).
Gobel, V., Barrett, P. L., Hall, D. H. & Fleming, J. T. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev. Cell 6, 865–873 (2004).
Van Furden, D., Johnson, K., Segbert, C. & Bossinger, O. The C. elegans ezrin-radixin-moesin protein ERM-1 is necessary for apical junction remodelling and tubulogenesis in the intestine. Dev. Biol. 272, 262–276 (2004).
Khan, L. A. et al. Intracellular lumen extension requires ERM-1-dependent apical membrane expansion and AQP-8-mediated flux. Nat. Cell Biol. 15, 143–156 (2013).
Baas, A. F. et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell 116, 457–466 (2004).
Rodriguez-Boulan, E. & Macara, I. G. Organization and execution of the epithelial polarity programme. Nat. Rev. Mol. Cell Biol. 15, 225–242 (2014).
Mooseker, M. S. & Cheney, R. E. Unconventional myosins. Annu. Rev. Cell Dev. Biol. 11, 633–675 (1995).
Tyska, M. J. & Mooseker, M. S. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys. J. 82, 1869–1883 (2002).
Tyska, M. J. & Mooseker, M. S. A role for myosin-1A in the localization of a brush border disaccharidase. J. Cell Biol. 165, 395–405 (2004).
Loomis, P. A. et al. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J. Cell Biol. 163, 1045–1055 (2003).
Zheng, L. et al. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell 102, 377–385 (2000).
Revenu, C. et al. A new role for the architecture of microvillar actin bundles in apical retention of membrane proteins. Mol. Biol. Cell 23, 324–336 (2012).
Zwaenepoel, I. et al. Ezrin regulates microvillus morphogenesis by promoting distinct activities of Eps8 proteins. Mol. Biol. Cell 23, 1080–1094 (2012).
Crawley, S. W. et al. Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell 157, 433–446 (2014).
Atuma, C., Strugala, V., Allen, A. & Holm, L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280, G922–G929 (2001).
McGuckin, M. A., Linden, S. K., Sutton, P. & Florin, T. H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 9, 265–278 (2011).
Fath, K. R., Mamajiwalla, S. N. & Burgess, D. R. The cytoskeleton in development of epithelial cell polarity. J. Cell Sci. Suppl. 17, 65–73 (1993).
Hirokawa, N., Tilney, L. G., Fujiwara, K. & Heuser, J. E. Organization of actin, myosin, and intermediate filaments in the brush border of intestinal epithelial cells. J. Cell Biol. 94, 425–443 (1982).
Moll, R. et al. The human gene encoding cytokeratin 20 and its expression during fetal development and in gastrointestinal carcinomas. Differentiation 53, 75–93 (1993).
Salas, P. J. Insoluble γ-tubulin-containing structures are anchored to the apical network of intermediate filaments in polarized CACO-2 epithelial cells. J. Cell Biol. 146, 645–658 (1999).
Salas, P. J., Rodriguez, M. L., Viciana, A. L., Vega-Salas, D. E. & Hauri, H. P. The apical submembrane cytoskeleton participates in the organization of the apical pole in epithelial cells. J. Cell Biol. 137, 359–375 (1997).
Ameen, N. A., Figueroa, Y. & Salas, P. J. Anomalous apical plasma membrane phenotype in CK8-deficient mice indicates a novel role for intermediate filaments in the polarization of simple epithelia. J. Cell Sci. 114, 563–575 (2001).
Owens, D. W. et al. Human keratin 8 mutations that disturb filament assembly observed in inflammatory bowel disease patients. J. Cell Sci. 117, 1989–1999 (2004).
Peterson, M. D., Bement, W. M. & Mooseker, M. S. An in vitro model for the analysis of intestinal brush border assembly. II. Changes in expression and localization of brush border proteins during cell contact-induced brush border assembly in Caco-2BBe cells. J. Cell Sci. 105, 461–472 (1993).
Heintzelman, M. B., Hasson, T. & Mooseker, M. S. Multiple unconventional myosin domains of the intestinal brush border cytoskeleton. J. Cell Sci. 107, 3535–3543 (1994).
Bazellieres, E. et al. Apico-basal elongation requires a drebrin-E–EB3 complex in columnar human epithelial cells. J. Cell Sci. 125, 919–931 (2012).
Lecuit, T. & Yap, A. S. E-cadherin junctions as active mechanical integrators in tissue dynamics. Nat. Cell Biol. 17, 533–539 (2015).
Mooseker, M. S. Brush border motility. Microvillar contraction in triton-treated brush borders isolated from intestinal epithelium. J. Cell Biol. 71, 417–433 (1976).
Keller, T. C. 3rd & Mooseker, M. S. Ca++-calmodulin-dependent phosphorylation of myosin, and its role in brush border contraction in vitro. J. Cell Biol. 95, 943–959 (1982).
Keller, T. C. 3rd, Conzelman, K. A., Chasan, R. & Mooseker, M. S. Role of myosin in terminal web contraction in isolated intestinal epithelial brush borders. J. Cell Biol. 100, 1647–1655 (1985).
Klingner, C. et al. Isotropic actomyosin dynamics promote organization of the apical cell cortex in epithelial cells. J. Cell Biol. 207, 107–121 (2014).
Makarova, O., Roh, M. H., Liu, C. J., Laurinec, S. & Margolis, B. Mammalian Crumbs3 is a small transmembrane protein linked to protein associated with Lin-7 (Pals1). Gene 302, 21–29 (2003).
Roh, M. H., Fan, S., Liu, C. J. & Margolis, B. The Crumbs3–Pals1 complex participates in the establishment of polarity in mammalian epithelial cells. J. Cell Sci. 116, 2895–2906 (2003).
Lemmers, C. et al. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol. Biol. Cell 15, 1324–1333 (2004).
Whiteman, E. L. et al. Crumbs3 is essential for proper epithelial development and viability. Mol. Cell. Biol. 34, 43–56 (2014).
Reifen, R. M., Cutz, E., Griffiths, A. M., Ngan, B. Y. & Sherman, P. M. Tufting enteropathy: a newly recognized clinicopathological entity associated with refractory diarrhea in infants. J. Pediatr. Gastroenterol. Nutr. 18, 379–385 (1994).
Goulet, O. et al. Intractable diarrhea of infancy with epithelial and basement membrane abnormalities. J. Pediatr. 127, 212–219 (1995).
Salomon, J. et al. Genetic characterization of congenital tufting enteropathy: epcam associated phenotype and involvement of SPINT2 in the syndromic form. Hum. Genet. 133, 299–310 (2014).
Maghzal, N., Kayali, H. A., Rohani, N., Kajava, A. V. & Fagotto, F. EpCAM controls actomyosin contractility and cell adhesion by direct inhibition of PKC. Dev. Cell 27, 263–277 (2013).
Vacca, B. et al. Drebrin E depletion in human intestinal epithelial cells mimics Rab8a loss of function. Hum. Mol. Genet. 23, 2834–2846 (2014).
Goulet, O., Salomon, J., Ruemmele, F., de Serres, N. P. & Brousse, N. Intestinal epithelial dysplasia (tufting enteropathy). Orphanet J. Rare Dis. 2, 20 (2007).
Kawaguchi, T. et al. Purification and cloning of hepatocyte growth factor activator inhibitor type 2, a Kunitz-type serine protease inhibitor. J. Biol. Chem. 272, 27558–27564 (1997).
Marlor, C. W. et al. Identification and cloning of human placental bikunin, a novel serine protease inhibitor containing two Kunitz domains. J. Biol. Chem. 272, 12202–12208 (1997).
Patey, N. et al. Distribution of cell adhesion molecules in infants with intestinal epithelial dysplasia (tufting enteropathy). Gastroenterology 113, 833–843 (1997).
Slae, M. A. et al. Syndromic congenital diarrhea because of the SPINT2 mutation showing enterocyte tufting and unique electron microscopy findings. Clin. Dysmorphol. 22, 118–120 (2013).
Davidson, G. P., Cutz, E., Hamilton, J. R. & Gall, D. G. Familial enteropathy: a syndrome of protracted diarrhea from birth, failure to thrive, and hypoplastic villus atrophy. Gastroenterology 75, 783–790 (1978).
Cutz, E. et al. Microvillus inclusion disease: an inherited defect of brush-border assembly and differentiation. N. Engl. J. Med. 320, 646–651 (1989).
Ameen, N. A. & Salas, P. J. Microvillus inclusion disease: a genetic defect affecting apical membrane protein traffic in intestinal epithelium. Traffic 1, 76–83 (2000).
Ruemmele, F. M., Schmitz, J. & Goulet, O. Microvillous inclusion disease (microvillous atrophy). Orphanet J. Rare Dis. 1, 22 (2006).
Sato, T. et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature 448, 366–369 (2007).
Muller, T. et al. MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat. Genet. 40, 1163–1165 (2008).
van der Velde, K. J. et al. An overview and online registry of microvillus inclusion disease patients and their MYO5B mutations. Hum. Mutat. 34, 1597–1605 (2013).
Roland, J. T. et al. Rab GTPase–Myo5B complexes control membrane recycling and epithelial polarization. Proc. Natl Acad. Sci. USA 108, 2789–2794 (2011).
Sobajima, T. et al. Rab11a is required for apical protein localisation in the intestine. Biol. Open 4, 86–94 (2014).
Knowles, B. C. et al. Rab11a regulates syntaxin 3 localization and microvillus assembly in enterocytes. J. Cell Sci. 128, 1617–1626 (2015).
Melendez, J. et al. Cdc42 coordinates proliferation, polarity, migration, and differentiation of small intestinal epithelial cells in mice. Gastroenterology 145, 808–819 (2013).
Knowles, B. C. et al. Myosin Vb uncoupling from RAB8A and RAB11A elicits microvillus inclusion disease. J. Clin. Invest. 124, 2947–2962 (2014).
Erickson, R. P., Larson-Thome, K., Valenzuela, R. K., Whitaker, S. E. & Shub, M. D. Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am. J. Med. Genet. A 146A, 3117–3119 (2008).
Raafat, F., Green, N. J., Nathavitharana, K. A. & Booth, I. W. Intestinal microvillous dystrophy: a variant of microvillous inclusion disease or a new entity? Hum. Pathol. 25, 1243–1248 (1994).
Mierau, G. W., Wills, E. J., Wyatt-Ashmead, J., Hoffenberg, E. J. & Cutz, E. Microvillous inclusion disease: report of a case with atypical features. Ultrastruct. Pathol. 25, 517–521 (2001).
Weeks, D. A., Zuppan, C. W., Malott, R. L. & Mierau, G. W. Microvillous inclusion disease with abundant vermiform, electron-lucent vesicles. Ultrastruct. Pathol. 27, 337–340 (2003).
Wiegerinck, C. L. et al. Loss of syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology 147, 65–68.e10 (2014).
Janecke, A. R. et al. Reduced sodium/proton exchanger NHE3 activity causes congenital sodium diarrhea. Hum. Mol. Genet. 24, 6614–6623 (2015).
Muller, T. et al. Congenital secretory diarrhoea caused by activating germline mutations in GUCY2C. Gut http://dx.doi.org/10.1136/gutjnl-2015-309441 (2015).
Sullivan, S. et al. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: potential contributors to IBD-associated diarrhea. Inflamm. Bowel Dis. 15, 261–274 (2009).
Chen, T. et al. Myosin VI mediates the movement of NHE3 down the microvillus in intestinal epithelial cells. J. Cell Sci. 127, 3535–3545 (2014).
Ubelmann, F. et al. Enterocyte loss of polarity and gut wound healing rely upon the F-actin-severing function of villin. Proc. Natl Acad. Sci. USA 110, E1380–E1389 (2013).
Thiery, J. P. & Sleeman, J. P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 (2006).
Chanrion, M. et al. Concomitant Notch activation and p53 deletion trigger epithelial-to-mesenchymal transition and metastasis in mouse gut. Nat. Commun. 5, 5005 (2014).
Lichtenberger, L. M. The hydrophobic barrier properties of gastrointestinal mucus. Annu. Rev. Physiol. 57, 565–583 (1995).
Tremaroli, V. & Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 (2012).
Pelaseyed, T. et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 260, 8–20 (2014).
Klaasen, H. L. et al. Intestinal, segmented, filamentous bacteria in a wide range of vertebrate species. Lab. Anim. 27, 141–150 (1993).
Yin, Y. et al. Comparative analysis of the distribution of segmented filamentous bacteria in humans, mice and chickens. ISME J. 7, 615–621 (2013).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Gaboriau-Routhiau, V. et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31, 677–689 (2009).
Chen, C. C., Baylor, M. & Bass, D. M. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology 105, 84–92 (1993).
Wikman, A., Karlsson, J., Carlstedt, I. & Artursson, P. A drug absorption model based on the mucus layer producing human intestinal goblet cell line HT29-H. Pharm. Res. 10, 843–852 (1993).
Iiboshi, Y. et al. Adhesive mucous gel layer and mucus release as intestinal barrier in rats. JPEN J. Parenter. Enteral Nutr. 20, 98–104 (1996).
McAuley, J. L. et al. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 117, 2313–2324 (2007).
Bates, J. M., Akerlund, J., Mittge, E. & Guillemin, K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe 2, 371–382 (2007).
Goldberg, R. F. et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc. Natl Acad. Sci. USA 105, 3551–3556 (2008).
Shifrin, D. A. Jr et al. Enterocyte microvillus-derived vesicles detoxify bacterial products and regulate epithelial–microbial interactions. Curr. Biol. 22, 627–631 (2012).
McConnell, R. E. et al. The enterocyte microvillus is a vesicle-generating organelle. J. Cell Biol. 185, 1285–1298 (2009).
Finlay, B. B. & Falkow, S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J. Infect. Dis. 162, 1096–1106 (1990).
Finlay, B. B., Ruschkowski, S. & Dedhar, S. Cytoskeletal rearrangements accompanying salmonella entry into epithelial cells. J. Cell Sci. 99, 283–296 (1991).
Francis, C. L., Ryan, T. A., Jones, B. D., Smith, S. J. & Falkow, S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364, 639–642 (1993).
Karunasagar, I., Senghaas, B., Krohne, G. & Goebel, W. Ultrastructural study of Listeria monocytogenes entry into cultured human colonic epithelial cells. Infect. Immun. 62, 3554–3558 (1994).
Ray, K., Marteyn, B., Sansonetti, P. J. & Tang, C. M. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat. Rev. Microbiol. 7, 333–340 (2009).
Ly, K. T. & Casanova, J. E. Mechanisms of Salmonella entry into host cells. Cell. Microbiol. 9, 2103–2111 (2007).
Lhocine, N. et al. Apical invasion of intestinal epithelial cells by Salmonella typhimurium requires villin to remodel the brush border actin cytoskeleton. Cell Host Microbe 17, 164–177 (2015).
Zhou, D., Mooseker, M. S. & Galan, J. E. An invasion-associated Salmonella protein modulates the actin-bundling activity of plastin. Proc. Natl Acad. Sci. USA 96, 10176–10181 (1999).
Frankel, G. et al. Generation of Escherichia coli intimin derivatives with differing biological activities using site-directed mutagenesis of the intimin C-terminus domain. Mol. Microbiol. 29, 559–570 (1998).
Shifrin, D. A. Jr, Crawley, S. W., Grega-Larson, N. E. & Tyska, M. J. Dynamics of brush border remodeling induced by enteropathogenic E. coli. Gut Microbes 5, 504–516 (2014).
Taylor, K. A., Luther, P. W. & Donnenberg, M. S. Expression of the EspB protein of enteropathogenic Escherichia coli within HeLa cells affects stress fibers and cellular morphology. Infect. Immun. 67, 120–125 (1999).
Iizumi, Y. et al. The enteropathogenic E. coli effector EspB facilitates microvillus effacing and antiphagocytosis by inhibiting myosin function. Cell Host Microbe 2, 383–392 (2007).
Mattoo, S., Alto, N. M. & Dixon, J. E. Subversion of myosin function by E. coli. Dev. Cell 14, 8–10 (2008).
Freeman, N. L. et al. Interaction of the enteropathogenic Escherichia coli protein, translocated intimin receptor (Tir), with focal adhesion proteins. Cell Motil. Cytoskeleton 47, 307–318 (2000).
Goosney, D. L. et al. Enteropathogenic E. coli translocated intimin receptor, Tir, interacts directly with α-actinin. Curr. Biol. 10, 735–738 (2000).
Cantarelli, V. V. et al. Cortactin is necessary for F-actin accumulation in pedestal structures induced by enteropathogenic Escherichia coli infection. Infect. Immun. 70, 2206–2209 (2002).
Cantarelli, V. V. et al. Cortactin is essential for F-actin assembly in enteropathogenic Escherichia coli (EPEC)- and enterohaemorrhagic E. coli (EHEC)-induced pedestals and the α-helical region is involved in the localization of cortactin to bacterial attachment sites. Cell. Microbiol. 8, 769–780 (2006).
Gruenheid, S. et al. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nat. Cell Biol. 3, 856–859 (2001).
Bommarius, B. et al. Enteropathogenic Escherichia coli Tir is an SH2/3 ligand that recruits and activates tyrosine kinases required for pedestal formation. Mol. Microbiol. 63, 1748–1768 (2007).
Nieto-Pelegrin, E., Kenny, B. & Martinez-Quiles, N. Nck adaptors, besides promoting N-WASP mediated actin-nucleation activity at pedestals, influence the cellular levels of enteropathogenic Escherichia coli Tir effector. Cell Adh. Migr. 8, 404–417 (2014).
Goluszko, P. et al. Decay-accelerating factor and cytoskeleton redistribution pattern in HeLa cells infected with recombinant Escherichia coli strains expressing Dr family of adhesins. Infect. Immun. 67, 3989–3997 (1999).
Peiffer, I. et al. Structural and functional lesions in brush border of human polarized intestinal Caco-2/TC7 cells infected by members of the Afa/Dr diffusely adhering family of Escherichia coli. Infect. Immun. 68, 5979–5990 (2000).
Van Loy, C. P., Sokurenko, E. V. & Moseley, S. L. The major structural subunits of Dr and F1845 fimbriae are adhesins. Infect. Immun. 70, 1694–1702 (2002).
Mounier, J., Vasselon, T., Hellio, R., Lesourd, M. & Sansonetti, P. J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect. Immun. 60, 237–248 (1992).
Scott, K. G., Yu, L. C. & Buret, A. G. Role of CD8+ and CD4+ T lymphocytes in jejunal mucosal injury during murine giardiasis. Infect. Immun. 72, 3536–3542 (2004).
Troeger, H. et al. Effect of chronic Giardia lamblia infection on epithelial transport and barrier function in human duodenum. Gut 56, 328–335 (2007).
Lauwaet, T. et al. Proteinase inhibitors TPCK and TLCK prevent Entamoeba histolytica induced disturbance of tight junctions and microvilli in enteric cell layers in vitro. Int. J. Parasitol. 34, 785–794 (2004).
Moser, L. A., Carter, M. & Schultz-Cherry, S. Astrovirus increases epithelial barrier permeability independently of viral replication. J. Virol. 81, 11937–11945 (2007).
Mendez, E. et al. Characterization of human astrovirus cell entry. J. Virol. 88, 2452–2460 (2014).
Chen, Y. Q. et al. The effect of enterohemorrhagic E. coli infection on the cell mechanics of host cells. PLoS ONE 9, e112137 (2014).
Van der Sluis, M. et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 (2006).
Heazlewood, C. K. et al. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 5, e54 (2008).
Sheng, Y. H. et al. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 6, 557–568 (2013).
Sheng, Y. H. et al. The MUC13 cell-surface mucin protects against intestinal inflammation by inhibiting epithelial cell apoptosis. Gut 60, 1661–1670 (2011).
Murata, Y. et al. Protein tyrosine phosphatase SAP-1 protects against colitis through regulation of CEACAM20 in the intestinal epithelium. Proc. Natl Acad. Sci. USA 112, E4264–E4271 (2015).
Kobayashi, I. et al. Autoantibodies to villin occur frequently in IPEX, a severe immune dysregulation, syndrome caused by mutation of FOXP3. Clin. Immunol. 141, 83–89 (2011).
DuPont, A. W. & DuPont, H. L. The intestinal microbiota and chronic disorders of the gut. Nat. Rev. Gastroenterol. Hepatol. 8, 523–531 (2011).
Jain, N. & Walker, W. A. Diet and host-microbial crosstalk in postnatal intestinal immune homeostasis. Nat. Rev. Gastroenterol. Hepatol. 12, 14–25 (2015).
Poley, J. R. Loss of the glycocalyx of enterocytes in small intestine: a feature detected by scanning electron microscopy in children with gastrointestinal intolerance to dietary protein. J. Pediatr. Gastroenterol. Nutr. 7, 386–394 (1988).
Kuitunen, P., Rapola, J., Savilahti, E. & Visakorpi, J. K. Response of the jejunal mucosa to cow's milk in the malabsorption syndrome with cow's milk intolerance. A light- and electron-microscopic study. Acta Paediatr. Scand. 62, 585–595 (1973).
Iancu, T. C. & Manov, I. Ultrastructural aspects of enterocyte defects in infancy and childhood. Ultrastruct. Pathol. 34, 117–125 (2010).
Shiner, M. & Birbeck, M. S. The microvilli of the small intestinal surface epithelium in coeliac disease and in idiopathic steatorrhoea. Gut 2, 277–284 (1961).
Magliocca, F. M. et al. Scanning electron microscopy of the small intestine during gluten-challenge in celiac disease. Arch. Histol. Cytol. 55 (Suppl.), 125–130 (1992).
Rostami, K. & Villanacci, V. Microscopic enteritis: novel prospect in coeliac disease clinical and immuno-histogenesis. Evolution in diagnostic and treatment strategies. Dig. Liver Dis. 41, 245–252 (2009).
Mine, Y. & Zhang, J. W. Surfactants enhance the tight-junction permeability of food allergens in human intestinal epithelial Caco-2 cells. Int. Arch. Allergy Immunol. 130, 135–142 (2003).
Secondulfo, M. et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig. Liver Dis. 36, 35–45 (2004).
Hope, H. B. et al. Small intestinal malabsorption in chronic alcoholism determined by 13C-D-xylose breath test and microscopic examination of the duodenal mucosa. Scand. J. Gastroenterol. 45, 39–45 (2010).
O'Brien, L. E., Zegers, M. M. & Mostov, K. E. Building epithelial architecture: insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol. 3, 531–537 (2002).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Sato, T. & Clevers, H. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340, 1190–1194 (2013).
McCracken, K. W., Howell, J. C., Wells, J. M. & Spence, J. R. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat. Protoc. 6, 1920–1928 (2011).
Spence, J. R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
Fordham, R. P. et al. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell 13, 734–744 (2013).
Wells, J. M. & Spence, J. R. How to make an intestine. Development 141, 752–760 (2014).
le Digabel, J., Ghibaudo, M., Trichet, L., Richert, A. & Ladoux, B. Microfabricated substrates as a tool to study cell mechanotransduction. Med. Biol. Eng. Comput. 48, 965–976 (2010).
Sung, J. H., Yu, J., Luo, D., Shuler, M. L. & March, J. C. Microscale 3D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab Chip 11, 389–392 (2011).
Huh, D. et al. Microfabrication of human organs-on-chips. Nat. Protoc. 8, 2135–2157 (2013).
Pearl, M., Fishkind, D., Mooseker, M., Keene, D. & Keller, T. 3rd Studies on the spectrin-like protein from the intestinal brush border, TW 260/240, and characterization of its interaction with the cytoskeleton and actin. J. Cell Biol. 98, 66–78 (1984).
Gunning, P. W., Hardeman, E. C., Lappalainen, P. & Mulvihill, D. P. Tropomyosin — master regulator of actin filament function in the cytoskeleton. J. Cell Sci. 128, 2965–2974 (2015).
Oriolo, A. S., Wald, F. A., Ramsauer, V. P. & Salas, P. J. Intermediate filaments: a role in epithelial polarity. Exp. Cell Res. 313, 2255–2264 (2007).
Schnell, U., Cirulli, V. & Giepmans, B. N. EpCAM: structure and function in health and disease. Biochim. Biophys. Acta 1828, 1989–2001 (2013).
Sivagnanam, M. et al. Identification of EpCAM as the gene for congenital tufting enteropathy. Gastroenterology 135, 429–437 (2008).
Sivagnanam, M. et al. Case of syndromic tufting enteropathy harbors SPINT2 mutation seen in congenital sodium diarrhea. Clin. Dysmorphol. 19, 48 (2010).
Tocchetti, A. et al. Loss of the actin remodeler Eps8 causes intestinal defects and improved metabolic status in mice. PLoS ONE 5, e9468 (2010).
Acknowledgements
The authors are supported by grants from the GEFLUC Paris-Ile de France “Les entreprises contre le cancer” (to D.D.), from “Initiatives d'excellence» (Idex ANR-11-IDEX-0005-02) - Labex Who Am I? (ANR-11-LABX-0071) (to D.D.), from “Postes accueil AP-HP – CNRS” (PhD fellowhip for to J.S.) and from the “Assistance Publique – Hôpitaux de Paris, AP-HP” (Projets Hospitaliers de Recherche Clinique (PHRC) DEI (#AOM07059) and AMVILLO (#AOM09136) grants, to D.D. in collaboration with O. Goulet).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects in the production of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Delacour, D., Salomon, J., Robine, S. et al. Plasticity of the brush border — the yin and yang of intestinal homeostasis. Nat Rev Gastroenterol Hepatol 13, 161–174 (2016). https://doi.org/10.1038/nrgastro.2016.5
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2016.5
This article is cited by
-
The nuclear receptor HNF4 drives a brush border gene program conserved across murine intestine, kidney, and embryonic yolk sac
Nature Communications (2021)
-
Mucin-2 knockout is a model of intercellular junction defects, mitochondrial damage and ATP depletion in the intestinal epithelium
Scientific Reports (2020)
-
Vps34/PI3KC3 deletion in kidney proximal tubules impairs apical trafficking and blocks autophagic flux, causing a Fanconi-like syndrome and renal insufficiency
Scientific Reports (2018)
-
Uptake of label-free graphene oxide by Caco-2 cells is dependent on the cell differentiation status
Journal of Nanobiotechnology (2017)
-
Intestinal transepithelial permeability of oxytocin into the blood is dependent on the receptor for advanced glycation end products in mice
Scientific Reports (2017)