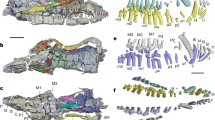
Abstract
A key transformation in mammalian ear evolution was incorporation of the primary jaw joint of premammalian synapsids into the definitive mammalian middle ear of living mammals. This evolutionary transition occurred in two steps, starting with a partial or ‘transitional’ mammalian middle ear in which the ectotympanic and malleus were still connected to the mandible by an ossified Meckel’s cartilage (MC), as observed in many Mesozoic mammals. This was followed by MC breakdown, freeing the ectotympanic and the malleus from the mandible and creating the definitive mammalian middle ear. Here we report new findings on the role of chondroclasts in MC breakdown, shedding light on how therian mammals lost the part of the MC connecting the ear to the jaw. Genetic or pharmacological loss of clast cells in mice and opossums leads to persistence of embryonic MC beyond juvenile stages, with MC ossification in mutant mice. The persistent MC causes a distinctive groove on the postnatal mouse dentary. This morphology phenocopies the ossified MC and Meckelian groove observed in Mesozoic mammals. Clast cell recruitment to MC is not observed in reptiles, where MC persists as a cartilaginous structure. We hypothesize that ossification of MC is an ancestral feature of mammaliaforms, and that a shift in the timing of clast cell recruitment to MC prior to its ossification is a key developmental mechanism for the evolution of the definitive mammalian middle ear in extant therians.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Allin, E. F. & Hopson, J. A. in The Evolutionary Biology of Hearing (eds Webster, D. B., Fay, R. R. & Popper, A. N. ) 587–614 (Springer, 1992).
Rowe, T. Coevolution of the mammalian middle ear and neocortex. Science 273, 651–654 (1996).
Luo, Z.-X. Transformation and diversification in early mammal evolution. Nature 450, 1011–1019 (2007).
Anthwal, N. & Tucker, A. S. in From Clone to Bone: The Synergy of Morphological and Molecular Tools in Palaeobiology (eds Asher, R. J. & Müller, J. ) 207–229 (Cambridge Univ. Press, 2012).
Maier, W. & Ruf, I. Evolution of the mammalian middle ear: a historical review. J. Anat. 228, 270–283 (2016).
Luo, Z.-X. Developmental patterns in Mesozoic evolution of mammal ears. Annu. Rev. Ecol. Evol. Syst. 42, 355–380 (2011).
Meng, J., Hu, Y., Wang, Y. & Li, C. The ossified Meckel’s cartilage and internal groove in Mesozoic mammaliaforms: implications to origin of the definitive mammalian middle ear. Zool. J. Linn. Soc. 138, 431–448 (2003).
Ji, Q., Luo, Z.-X., Zhang, X., Yuan, C.-X. & Xu, L. Evolutionary development of the middle ear in Mesozoic therian mammals. Science 326, 278–281 (2009).
Takechi, M. & Kuratani, S. History of studies on mammalian middle ear evolution: a comparative morphological and developmental biology perspective. J. Exp. Zool. B 314, 417–433 (2010).
Amano, O. et al. Meckel’s cartilage: discovery, embryology and evolution. J. Oral Biosci. 52, 125–135 (2010).
Anthwal, N., Joshi, L. & Tucker, A. S. Evolution of the mammalian middle ear and jaw: adaptations and novel structures. J. Anat. 222, 147–160 (2013).
Sánchez-Villagra, M. R., Gemballa, S., Nummela, S., Smith, K. K. & Maier, W. Ontogenetic and phylogenetic transformations of the ear ossicles in marsupial mammals. J. Morphol. 251, 219–238 (2002).
Amin, S. & Tucker, A. S. Joint formation in the middle ear: lessons from the mouse and guinea pig. Dev. Dyn. 235, 1326–1333 (2006).
Wilson, J. & Tucker, A. S. Fgf and Bmp signals repress the expression of Bapx1 in the mandibular mesenchyme and control the position of the developing jaw joint. Dev. Biol. 266, 138–150 (2004).
Meng, J., Wang, Y. & Li, C. Transitional mammalian middle ear from a new Cretaceous Jehol eutriconodont. Nature 472, 181–185 (2011).
Luo, Z.-X., Gatesy, S. M., Jenkins, F. A., Amaral, W. W. & Shubin, N. H. Mandibular and dental characteristics of Late Triassic mammaliaform Haramiyavia and their ramifications for basal mammal evolution. Proc. Natl Acad. Sci. USA 112, E7101–E7109 (2015).
Holliday, C. M. & Nesbitt, S. J. Morphology and diversity of the mandibular symphysis of archosauriforms. Geol. Soc. Lond. Spec. Publ. 379, 555–571 (2013).
Smith, K. K. Craniofacial development in marsupial mammals: developmental origins of evolutionary change. Dev. Dyn. 235, 1181–1193 (2006).
Gaupp, E. W. T. Die Reichertsche theorie: (Hammer-, amboss-und kieferfrage). Arch. Anat. Suppl. 1912, 1–416 (1913).
Hall, B. K. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. (Academic, 2015).
Knowles, H. J. et al. Chondroclasts are mature osteoclasts which are capable of cartilage matrix resorption. Virchows Arch. 461, 205–210 (2012).
Lewinson, D. & Silbermann, M. Chondroclasts and endothelial cells collaborate in the process of cartilage resorption. Anat. Rec. 233, 504–514 (1992).
Grigoriadis, A. et al. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science 266, 443–448 (1994).
Arai, A. et al. Fos plays an essential role in the upregulation of RANK expression in osteoclast precursors within the bone microenvironment. J. Cell Sci. 125, 2910–2917 (2012).
Rogers, M. J., Crockett, J. C., Coxon, F. P. & Mönkkönen, J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 49, 34–41 (2011).
Martin, T. et al. A Cretaceous eutriconodont and integument evolution in early mammals. Nature 526, 380–384 (2015).
Raff, R. A. Written in stone: fossils, genes and evo–devo. Nat. Rev. Genet. 8, 911–920 (2007).
Urban, D. J. U. et al. A new developmental mechanism for the separation of the mammalian middle ear ossicles from the jaw. Proc. R. Soc. B. (in the press).
Sakakura, Y. Role of matrix metalloproteinases in extracellular matrix disintegration of Meckel’s cartilage in mice. J. Oral Biosci. 52, 143–149 (2010).
Yang, L., Tsang, K. Y., Tang, H. C., Chan, D. & Cheah, K. S. E. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc. Natl Acad. Sci. USA 111, 12097–12102 (2014).
Boyce, B. F. et al. New roles for osteoclasts in bone. Ann. NY Acad. Sci. 1116, 245–254 (2007).
Sakakura, Y. et al. Immunolocalization of receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoprotegerin (OPG) in Meckel’s cartilage compared with developing endochondral bones in mice. J. Anat. 207, 325–337 (2005).
Wang, Y., Zheng, Y., Chen, D. & Chen, Y. Enhanced BMP signaling prevents degeneration and leads to endochondral ossification of Meckel′s cartilage in mice. Dev. Biol. 381, 301–311 (2013).
Abzhanov, A. von Baer’s law for the ages: lost and found principles of developmental evolution. Trends Genet. 29, 712–722 (2013).
Wang, Z. Q. et al. Bone and haematopoietic defects in mice lacking c-fos. Nature 360, 741–745 (1992).
Popa, E. M., Anthwal, N. & Tucker, A. S. Complex patterns of tooth replacement revealed in the fruit bat (Eidolon helvum). J. Anat. (2016). E-pub ahead of print. http://dx.doi.org/10.1111/joa.12522
Ealba, E. L. et al. Neural crest-mediated bone resorption is a determinant of species-specific jaw length. Dev. Biol. 408, 151–163 (2015).
Acknowledgements
We thank C. Healy (KCL) and L. Yin (UIUC) for support with μCT, E. Popa (King’s College London, KCL) for assistance in processing tissue, J. M. Fons (KCL) for injection of newborn mice, A. Neander (University of Chicago) for graphics assistance, A. Grigoriadis (KCL) for supplying the c-Fos mutant mice and in situ probes, and J. Turner and F. Decarpentrie (Francis Crick Institute) for supplying opossum pups. For this project, N.A. was supported by the Leverhulme Trust (RPG-2013-070) and the Wellcome Trust (102889/Z/13/Z) and NSF/EDEN Research Exchange Grant (IOS 0955517) A.S.T. is funded by the Wellcome Trust (102889/Z/13/Z). D.J.U. was supported by a NSF Graduate Research Fellowship (2013136301) and K.E.S. by a Doctoral Dissertation Improvement Grant (1406802).
Author information
Authors and Affiliations
Contributions
N.A. and A.S.T. conceived and designed the project. N.A. carried out mouse and reptile experimental work; K.E.S. and D.J.U. carried out opossum experimental work. Z.X.L. carried out fossil analysis. N.A. wrote the manuscript with A.S.T. A.S.T., Z.X.L, K.E.S. and D.J.U. critically appraised and edited the manuscript. All authors read and approved the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures 1–6; Supplementary Discussion (PDF 8546 kb)
Rights and permissions
About this article
Cite this article
Anthwal, N., Urban, D., Luo, ZX. et al. Meckel’s cartilage breakdown offers clues to mammalian middle ear evolution. Nat Ecol Evol 1, 0093 (2017). https://doi.org/10.1038/s41559-017-0093
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-017-0093
This article is cited by
-
Fossils document evolutionary changes of jaw joint to mammalian middle ear
Nature (2024)
-
Middle ear innovation in Early Cretaceous eutherian mammals
Nature Communications (2023)
-
Altered developmental programs and oriented cell divisions lead to bulky bones during salamander limb regeneration
Nature Communications (2022)
-
Spatial and chronological localization of septoclasts in the mouse Meckel’s cartilage
Histochemistry and Cell Biology (2022)
-
Postnatal development in a marsupial model, the fat-tailed dunnart (Sminthopsis crassicaudata; Dasyuromorphia: Dasyuridae)
Communications Biology (2021)