Key Points
-
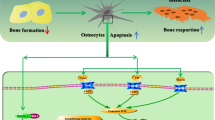
Osteocytes constitute >95% of the cells in bone
-
Osteocytes are differentiated osteoblasts that live within the bone matrix and are highly connected among themselves and with cells on the bone surface and the bone marrow
-
The molecular and functional signature of osteocytes comprises genes and proteins that control dendritic morphology and canaliculi formation, phosphate metabolism and matrix mineralization, bone formation and bone resorption
-
Osteocytes orchestrate the function of osteoblasts and osteoclasts in response to both mechanical and hormonal cues
-
Osteocytes produce and secrete factors (sclerostin, RANKL and OPG) that affect other bone cells by paracrine and/or autocrine mechanisms and secrete hormones (such as FGF23) that affect other tissues by endocrine mechanisms
-
Novel therapeutic approaches harness the accumulating knowledge of osteocyte biology, thus, targeting osteocytic signalling pathways and messengers to improve skeletal health
Abstract
Osteocytes are differentiated osteoblasts that become surrounded by matrix during the process of bone formation. Acquisition of the osteocyte phenotype is achieved by profound changes in gene expression that facilitate adaptation to the changing cellular environment and constitute the molecular signature of osteocytes. During osteocytogenesis, the expression of genes that are characteristic of the osteoblast are altered and the expression of genes and/or proteins that impart dendritic cellular morphology, regulate matrix mineralization and control the function of cells at the bone surface are ordely modulated. The discovery of mutations in human osteocytic genes has contributed, in a large part, to our understanding of the role of osteocytes in bone homeostasis. Osteocytes are targets of the mechanical force imposed on the skeleton and have a critical role in integrating mechanosensory pathways with the action of hormones, which thereby leads to the orchestrated response of bone to environmental cues. Current, therapeutic approaches harness this accumulating knowledge by targeting osteocytic signalling pathways and messengers to improve skeletal health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
29 July 2016
Nature Reviews Endocrinology 10.1038/nrendo.2016.71 In Figure 4 of the above article published online 27 May 2016, the label at the bottom of the right side of the figure should have read: Osteoblast generation/survival and bone formation/mineralization. This has been corrected in the online versions of the article.
References
Bonewald, L. F. The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 (2011). This review describes the process of osteocyte differentiation, the role of mechanosensory cells in the regulation of osteoclast function and mineral metabolism, and the importance of osteocyte viability.
Bellido, T. Osteocyte-driven bone remodeling. Calcif. Tissue Int. 94, 25–34 (2013). This review focuses on the role of osteocytes in the regulation of bone resorption and formation.
Marotti, G. & Palumbo, C. The mechanism of transduction of mechanical strains into biological signals at the bone cellular level. Eur. J. Histochem. 51 (Suppl. 1), 15–19 (2007). A discussion of the authors' investigations on osteocyte morphology and the functional implications of their findings.
Marotti, G. The structure of bone tissues and the cellular control of their deposition. Ital. J. Anat. Embryol. 101, 25–79 (1996).
Martin, R. B. Does osteocyte formation cause the nonlinear refilling of osteons? Bone 26, 71–78 (2000).
Frost, H. M. In vivo osteocyte death. J. Bone Joint Surg. Am. 42-A, 138–143 (1960).
Arnold, J. S., Frost, H. M. & Buss, R. O. The osteocyte as a bone pump. Clin. Orthop. Relat. Res. 78, 47–55 (1971).
Frost, H. M. Bone 'mass' and the 'mechanostat': a proposal. Anat. Rec. 219, 1–9 (1987).
Frost, H. M. Bone's mechanostat: a 2003 update. Anat. Rec. 275A, 1081–1101 (2003).
Robling, A. G. et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 283, 5866–5875 (2008).
Tu, X. et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 50, 209–217 (2012).
Parfitt, A. M. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone diseases. II. PTH and bone cells: bone turnover and plasma calcium regulation. Metabolism 25, 909–955 (1976).
O'Brien, C. A. et al. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS ONE 3, e2942 (2008).
Rhee, Y. et al. PTH receptor signaling in osteocytes governs periosteal bone formation and intra-cortical remodeling. J. Bone Miner. Res. 26, 1035–1046 (2011).
Rhee, Y. et al. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49, 636–643 (2011).
Rhee, Y. et al. Resorption controls bone anabolism driven by PTH receptor signaling in osteocytes. J. Biol. Chem. 288, 29809–29820 (2013).
Saini, V. et al. Parathyroid hormone (PTH)/PTH-related peptide type 1 receptor (PPR) signaling in osteocytes regulates anabolic and catabolic skeletal responses to PTH. J. Biol. Chem. 288, 20122–20134 (2013).
Tu, X. et al. Conditional deletion of the parathyroid hormone (PTH) receptor 1 from osteocytes results in decreased bone resorption and a progressive increase in cancellous bone mass. J. Bone Miner. Res. 26, S16 (2011).
Tu, X. et al. PTH receptor 1 expression in osteocytes is indispensable for the anabolic effect of mechanical loading in mice. J. Bone Miner. Res. 26, S24 (2011).
Qing, H. et al. Demonstration of osteocytic perilacunar/canalicular remodeling in mice during lactation. J. Bone Miner. Res. 27, 1018–1029 (2012).
Belanger, L. F. Osteocytic osteolysis. Calcif. Tissue Res. 4, 1–12 (1969).
Parfitt, A. M. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone disease. Part I of IV parts: mechanisms of calcium transfer between blood and bone and their cellular basis: morphological and kinetic approaches to bone turnover. Metabolism 25, 809–844 (1976).
Martin, R. B., Burr, D. B. & Sharkey, N. A. Skeletal Tissue Mechanics (Springer-Verlag, 1998).
Nose, K., Saito, H. & Kuroki, T. Isolation of a gene sequence induced later by tumor-promoting 12-O-tetradecanoylphorbol-13-acetate in mouse osteoblastic cells (MC3T3-E1) and expressed constitutively in ras-transformed cells. Cell Growth Differ. 1, 511–518 (1990).
Wetterwald, A. et al. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone 18, 125–132 (1996).
Boucherot, A., Schreiber, R., Pavenstadt, H. & Kunzelmann, K. Cloning and expression of the mouse glomerular podoplanin homologue gp38P. Nephrol. Dial. Transplant. 17, 978–984 (2002).
Rishi, A. K. et al. Cloning, characterization, and development expression of a rat lung alveolar type I cell gene in embryonic endodermal and neural derivatives. Dev. Biol. 167, 294–306 (1995).
Zhang, K. et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol. Cell. Biol. 26, 4539–4552 (2006).
Skupien, A. et al. CD44 regulates dendrite morphogenesis through Src tyrosine kinase-dependent positioning of the Golgi. J. Cell Sci. 127, 5038–5051 (2014).
Hughes, D. E., Salter, D. M. & Simpson, R. CD44 expression in human bone: a novel marker of osteocytic differentiation. J. Bone Miner. Res. 9, 39–44 (1994).
Ohizumi, I. et al. Association of CD44 with OTS-8 in tumor vascular endothelial cells. Biochim. Biophys. Acta 1497, 197–203 (2000).
Tanaka-Kamioka, K., Kamioka, H., Ris, H. & Lim, S. S. Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. J. Bone Miner. Res. 13, 1555–1568 (1998).
Kamioka, H., Sugawara, Y., Honjo, T., Yamashiro, T. & Takano-Yamamoto, T. Terminal differentiation of osteoblasts to osteocytes is accompanied by dramatic changes in the distribution of actin-binding proteins. J. Bone Miner. Res. 19, 471–478 (2004).
van Dijk, F. S. et al. PLS3 mutations in X-linked osteoporosis with fractures. N. Engl. J. Med. 369, 1529–1536 (2013).
Bellido, T., Plotkin, L. I. & Bruzzaniti, A. in Bone cells in Basic and Applied Bone Biology (eds Burr, D. & Allen, M.) 27–45 (Elsevier, 2014). This book chapter describes the current knowledge on the generation, survival and function of osteoblasts, osteocytes and osteoclasts.
Feng, J. Q. et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat. Genet. 38, 1310–1315 (2006).
Zhang, R. et al. Unique roles of phosphorus in endochondral bone formation and osteocyte maturation. J. Bone Miner. Res. 26, 1047–1056 (2011).
Ren, Y. et al. Sclerostin antibody (Scl-Ab) improves osteomalacia phenotype in dentin matrix 1 (Dmp1) knockout mice with little impact on serum levels of phosphorus and FGF23. Matrix Biol. http://dx.doi.org/10.1016/j.matbiol.2015.12.009 (2015).
Holmbeck, K. et al. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J. Cell Sci. 118, 147–156 (2005).
Zhao, W., Byrne, M. H., Wang, Y. & Krane, S. M. Osteocyte and osteoblast apoptosis and excessive bone deposition accompany failure of collagenase cleavage of collagen. J. Clin. Invest. 106, 941–949 (2000).
Plotkin, L. I. & Bellido, T. Beyond gap junctions: connexin43 and bone cell signaling. Bone 52, 157–166 (2013). This article discusses the evidence demonstrating that CX43 is a key component of the intracellular machinery responsible for signal transduction in bone cells.
Qiu, S., Rao, D. S., Palnitkar, S. & Parfitt, A. M. Age and distance from the surface but not menopause reduce osteocyte density in human cancellous bone. Bone 31, 313–318 (2002).
Almeida, M. et al. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J. Biol. Chem. 282, 27285–27297 (2007).
Bivi, N. et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J. Bone Miner. Res. 27, 374–389 (2012).
Davis, H. M. et al. Reduction in microRNA21 promotes apoptosis and increases RANKL in osteocytes: a mechanism for enhanced resorption in the absence of Cx43 in osteoblastic cells and with aging. J. Bone Miner. Res. 30, S101 (2015).
Plotkin, L. I., Manolagas, S. C. & Bellido, T. Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 277, 8648–8657 (2002).
Bivi, N., Lezcano, V., Romanello, M., Bellido, T. & Plotkin, L. I. Connexin43 interacts with βarrestin: a pre-requisite for osteoblast survival induced by parathyroid hormone. J. Cell. Biochem. 112, 2920–2930 (2011).
Chung, D. et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J. Cell Sci. 119, 4187–4198 (2006).
Pacheco-Costa, R. et al. Defective cancellous bone structure and abnormal response to PTH in cortical bone of mice lacking Cx43 cytoplasmic C-terminus domain. Bone 81, 632–643 (2015).
Plotkin, L. I., Speacht, T. L. & Donahue, H. J. Cx43 and mechanotransduction in bone. Curr. Osteoporos. Rep. 13, 67–72 (2015). This review describes the complex role of CX43 expressed in osteoblastic cells in the transduction of mechanical signals.
Grimston, S. K., Watkins, M. P., Brodt, M. D., Silva, M. J. & Civitelli, R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PLoS ONE 7, e44222 (2012).
Zhang, Y. et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE 6, e23516 (2011).
Bivi, N. et al. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J. Orthop. Res. 31, 1075–1081 (2013).
Mattinzoli, D. et al. FGF23-regulated production of Fetuin-A (AHSG) in osteocytes. Bone 83, 35–47 (2015).
Shimada, T. et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. USA 98, 6500–6505 (2001).
Sitara, D. et al. Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol. 23, 421–432 (2004).
Sitara, D. et al. Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet. 4, e1000154 (2008).
Feng, J. Q., Clinkenbeard, E. L., Yuan, B., White, K. E. & Drezner, M. K. Osteocyte regulation of phosphate homeostasis and bone mineralization underlies the pathophysiology of the heritable disorders of rickets and osteomalacia. Bone 54, 213–221 (2013).
Rowe, P. S. The chicken or the egg: PHEX, FGF23 and SIBLINGs unscrambled. Cell Biochem. Funct. 30, 355–375 (2012).
Gowen, L. C. et al. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J. Biol. Chem. 278, 1998–2007 (2003).
Harris, S. E. et al. DMP1 and MEPE expression are elevated in osteocytes after mechanical loading in vivo: theoretical role in controlling mineral quality in the perilacunar matrix. J. Musculoskelet. Neuronal Interact. 7, 313–315 (2007).
Rowe, P. S. Regulation of bone-renal mineral and energy metabolism: the PHEX, FGF23, DMP1, MEPE ASARM pathway. Crit. Rev. Eukaryot. Gene Expr. 22, 61–86 (2012).
Yuan, B. et al. Aberrant Phex function in osteoblasts and osteocytes alone underlies murine X-linked hypophosphatemia. J. Clin. Invest. 118, 722–734 (2008).
Brown, W. W. et al. Hypophosphatemia with elevations in serum fibroblast growth factor 23 in a child with Jansen's metaphyseal chondrodysplasia. J. Clin. Endocrinol. Metab. 94, 17–20 (2009).
Baron, R. & Kneissel, M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat. Med. 19, 179–192 (2013). A comprehensive review on the consequences of activating or inhibiting Wnt signalling in the skeleton, which summarizes the findings of research using genetically modified mice and pharmacological approaches, and the potential of manipulating the pathway for the treatment of bone diseases.
Paic, F. et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone 45, 682–692 (2009).
Li, J. et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39, 754–766 (2006).
Bodine, P. V. et al. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J. Cell. Biochem. 96, 1212–1230 (2005).
Poole, K. E. et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 19, 1842–1844 (2005).
Li, X. et al. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 280, 19883–19887 (2005).
Leupin, O. et al. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J. Biol. Chem. 286, 19489–19500 (2011).
Kim, S. J. et al. Identification of signal peptide domain SOST mutations in autosomal dominant craniodiaphyseal dysplasia. Hum. Genet. 129, 497–502 (2011).
Balemans, W. et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10, 537–543 (2001).
Li, X. et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner. Res. 23, 860–869 (2008).
Chang, M. K. et al. Disruption of Lrp4 function by genetic deletion or pharmacological blockade increases bone mass and serum sclerostin levels. Proc. Natl Acad. Sci. USA 111, E5187–E5195 (2014).
McClung, M. R. Emerging therapies for osteoporosis. Endocrinol. Metab. (Seoul.) 30, 429–435 (2015).
Niziolek, P. J. et al. High bone mass-causing mutant LRP5 receptors are resistant to endogenous inhibitors in vivo. J. Bone Miner. Res. 30, 1822–1830 (2015).
Kramer, I., Loots, G. G., Studer, A., Keller, H. & Kneissel, M. Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J. Bone Miner. Res. 25, 178–189 (2010).
Cui, Y. et al. Lrp5 functions in bone to regulate bone mass. Nat. Med. 17, 684–691 (2011).
Little, R. D. et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am. J. Hum. Genet. 70, 11–19 (2002).
Tu, X. et al. Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc. Natl Acad. Sci. USA 112, E478–E486 (2015). This study demonstrated the effect of activating canonical Wnt/ β -catenin signalling in osteocytes, leading to bone anabolism.
Ducy, P. et al. Increased bone formation in osteocalcin-deficient mice. Nature 382, 448–452 (1996).
Kato, Y., Windle, J. J., Koop, B. A., Mundy, G. R. & Bonewald, L. F. Establishment of an osteocyte-like cell line, MLO-Y4. J. Bone Miner. Res. 12, 2014–2023 (1997).
Confavreux, C. B., Levine, R. L. & Karsenty, G. A paradigm of integrative physiology, the crosstalk between bone and energy metabolisms. Mol. Cell. Endocrinol. 310, 21–29 (2009).
Oury, F. et al. Endocrine regulation of male fertility by the skeleton. Cell 144, 796–809 (2011).
Oury, F. et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell 155, 228–241 (2013).
Xiong, J. et al. Matrix-embedded cells control osteoclast formation. Nat. Med. 17, 1235–1241 (2011).
Nakashima, T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 17, 1231–1234 (2011).
Suda, T., Nakamura, I., Jimi, E. & Takahashi, N. Regulation of osteoclast function. J. Bone Miner. Res. 12, 869–879 (1997).
Harris, S. E. et al. Meox2Cre-mediated disruption of CSF-1 leads to osteopetrosis and osteocyte defects. Bone 50, 42–53 (2012).
Abboud-Werner, S. L. et al. CSF in osteocytes and late osteoblasts controls major aspects of bone remodeling. J. Bone Miner. Res. 28, S31 (2013).
Kramer, I. et al. Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol. Cell. Biol. 30, 3071–3085 (2010).
Holmen, S. L. et al. Essential role of β-catenin in postnatal bone acquisition. J. Biol. Chem. 280, 21162–21168 (2005).
Glass, D. A. et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 8, 751–764 (2005).
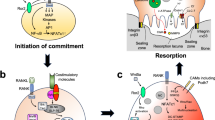
Plotkin, L. I. Apoptotic osteocytes and the control of targeted bone resorption. Curr. Osteoporos. Rep. 12, 121–126 (2014).
Schaffler, M. B., Cheung, W. Y., Majeska, R. & Kennedy, O. Osteocytes: master orchestrators of bone. Calcif. Tissue Int. 94, 5–24 (2013).
Tatsumi, S. et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 5, 464–475 (2007).
Plotkin, L. I. et al. Inhibition of osteocyte apoptosis prevents the increase in osteocytic receptor activator of nuclear factor κB ligand RANKL but it does not stop bone resorption or the loss of bone induced by unloading. J. Biol. Chem. 290, 18934–18941 (2015).
Cabahug-Zuckerman, P. et al. Osteocyte apoptosis caused by hindlimb unloading is required to trigger osteocyte RANKL production and subsequent resorption of cortical and trabecular bone in mice femurs. J. Bone Miner. Res. http://dx.doi.org/10.1002/jbmr.2807 (2016).
Aguirre, J. I. et al. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 21, 605–615 (2006).
Morse, L. R. et al. Severe spinal cord injury causes immediate multi-cellular dysfunction at the chondro-osseous junction. Transl. Stroke Res. 2, 643–650 (2011).
Noble, B. S. et al. Mechanical loading: biphasic osteocyte survival and the targeting of osteoclasts for bone destruction in rat cortical bone. Am. J. Physiol. Cell Physiol. 284, C934–C943 (2003).
Bellido, T. Antagonistic interplay between mechanical forces and glucocorticoids in bone: a tale of kinases. J. Cell. Biochem. 111, 1–6 (2010).
Plotkin, L. I. et al. Mechanical stimulation prevents osteocyte apoptosis: requirement of integrins Src kinases and ERKs. Am. J. Physiol. Cell Physiol. 289, C633–C643 (2005).
McNamara, L. M., Majeska, R. J., Weinbaum, S., Friedrich, V. & Schaffler, M. B. Attachment of osteocyte cell processes to the bone matrix. Anat. Rec. (Hoboken) 292, 355–363 (2009).
Wang, Y., McNamara, L. M., Schaffler, M. B. & Weinbaum, S. A model for the role of integrins in flow induced mechanotransduction in osteocytes. Proc. Natl Acad. Sci. USA 104, 15941–15946 (2007).
Phillips, J. A. et al. Role for β1 integrins in cortical osteocytes during acute musculoskeletal disuse. Matrix Biol. 27, 609–618 (2008).
Robinson, J. A. et al. WNT/β-catenin signaling is a normal physiological response to mechanical loading in bone. J. Biol. Chem. 281, 31720–31728 (2006).
Sutherland, M. K. et al. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone 35, 828–835 (2004).
Almeida, M., Han, L., Bellido, T., Manolagas, S. C. & Kousteni, S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J. Biol. Chem. 280, 41342–41351 (2005).
Gortazar, A. R., Martin-Millan, M., Bravo, B., Plotkin, L. I. & Bellido, T. Crosstalk between caveolin-1/ERKs and ß-catenin survival pathways in osteocyte mechanotransduction. J. Biol. Chem. 288, 8168–8175 (2013).
Kitase, Y., Barragan, L., Jiang, J. X., Johnson, M. L. & Bonewald, L. F. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid induced apoptosis through both the β-catenin and PKA pathways. J. Bone Miner. Res. 25, 2657–2668 (2010).
Babij, P. et al. High bone mass in mice expressing a mutant LRP5 gene. J. Bone Miner. Res. 18, 960–974 (2003).
Santos, A., Bakker, A. D. & Klein-Nulend, J. The role of osteocytes in bone mechanotransduction. Osteoporos. Int. 20, 1027–1031 (2009).
Sawakami, K. et al. The WNT co-receptor LRP5 is essential for skeletal mechanotransduction, but not for the anabolic bone response to parathyroid hormone treatment. J. Biol. Chem. 281, 23698–23711 (2006).
Chow, J. W. & Chambers, T. J. Indomethacin has distinct early and late actions on bone formation induced by mechanical stimulation. Am. J. Physiol. 267, E287–E292 (1994).
Kousteni, S. et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104, 719–730 (2001). This study demonstrated the anti-apoptotic effects of oestrogens and androgens on osteoblasts and osteocytes, and defined the signalling pathways activated by these hormones.
Aguirre, J. I. et al. A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J. Biol. Chem. 282, 25501–25508 (2007).
Jessop, H. L. et al. Mechanical strain and estrogen activate estrogen receptor α in bone cells. J. Bone Miner. Res. 16, 1045–1055 (2001).
Lee, K., Jessop, H., Suswillo, R., Zaman, G. & Lanyon, L. Endocrinology: bone adaptation requires oestrogen receptor-α. Nature 424, 389 (2003).
Lee, K. C., Jessop, H., Suswillo, R., Zaman, G. & Lanyon, L. E. The adaptive response of bone to mechanical loading in female transgenic mice is deficient in the absence of oestrogen receptor-α and -β. J. Endocrinol. 182, 193–201 (2004).
Bellido, T., Saini, V. & Divieti Pajevic, P. Effects of PTH on osteocyte function. Bone 54, 250–257 (2013).
Chow, J. W., Fox, S., Jagger, C. J. & Chambers, T. J. Role for parathyroid hormone in mechanical responsiveness of rat bone. Am. J. Physiol. 274, E146–E154 (1998).
Ma, Y. et al. Parathyroid hormone and mechanical usage have a synergistic effect in rat tibial diaphyseal cortical bone. J. Bone Miner. Res. 14, 439–448 (1999).
Hagino, H., Okano, T., Akhter, M. P., Enokida, M. & Teshima, R. Effect of parathyroid hormone on cortical bone response to in vivo external loading of the rat tibia. J. Bone Miner. Metab. 19, 244–250 (2001).
Sugiyama, T. et al. Mechanical loading enhances the anabolic effects of intermittent parathyroid hormone (1-34) on trabecular and cortical bone in mice. Bone 43, 238–248 (2008).
Gardinier, J. D., Mohamed, F. & Kohn, D. H. PTH signaling during exercise contributes to bone adaptation. J. Bone Miner. Res. 30, 1053–1063 (2015).
Li, J., Duncan, R. L., Burr, D. B., Gattone, V. H. & Turner, C. H. Parathyroid hormone enhances mechanically induced bone formation, possibly involving L-type voltage-sensitive calcium channels. Endocrinology 144, 1226–1233 (2003).
Wysolmerski, J. J. Parathyroid hormone-related protein: an update. J. Clin. Endocrinol. Metab. 97, 2947–2956 (2012).
Chen, X., Macica, C. M., Ng, K. W. & Broadus, A. E. Stretch-induced PTH-related protein gene expression in osteoblasts. J. Bone Miner. Res. 20, 1454–1461 (2005).
Maycas, M. et al. Role of the parathyroid hormone type 1 receptor (PTH1R) as a mechanosensor in osteocyte survival. J. Bone Miner. Res. 30, 1231–1244 (2015).
Weinstein, R. S. Clinical practice. Glucocorticoid-induced bone disease. N. Engl. J. Med. 365, 62–70 (2011).
Plotkin, L. I. et al. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Invest. 104, 1363–1374 (1999). This study showed that part of the beneficial effect of bisphosphonates on bone strength can be ascribed to the anti-apoptotic effect of the drugs on osteocytes and osteoblasts.
Weinstein, R. S., Jilka, R. L., Parfitt, A. M. & Manolagas, S. C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J. Clin. Invest. 102, 274–282 (1998). This report demonstrated the deleterious effect of therapeutic doses of glucocorticoids on the differentiation and viability of osteoblasts and osteocytes, and proposed that glucocorticoid- induced bone disease arises from changes in the numbers of bone cells.
Weinstein, R. S., Nicholas, R. W. & Manolagas, S. C. Apoptosis of osteocytes in glucocorticoid-induced osteonecrosis of the hip. J. Clin. Endocrinol. Metab. 85, 2907–2912 (2000).
Gu, G., Hentunen, T. A., Nars, M., Harkonen, P. L. & Vaananen, H. K. Estrogen protects primary osteocytes against glucocorticoid-induced apoptosis. Apoptosis 10, 583–595 (2005).
Plotkin, L. I., Manolagas, S. C. & Bellido, T. Glucocorticoids induce osteocyte apoptosis by blocking focal adhesion kinase-mediated survival: evidence for inside-out signaling leading to anoikis. J. Biol. Chem. 282, 24120–24130 (2007).
Gil-Henn, H. et al. Defective microtubule-dependent podosome organization in osteoclasts leads to increased bone density in Pyk2−/− mice. J. Cell Biol. 178, 1053–1064 (2007).
Buckbinder, L. et al. Proline-rich tyrosine kinase 2 regulates osteoprogenitor cells and bone formation, and offers an anabolic treatment approach for osteoporosis. Proc. Natl Acad. Sci. USA 104, 10619–10624 (2007).
Sato, A. Y., Tu, X., McAndrews, K. A., Plotkin, L. I. & Bellido, T. Prevention of glucocorticoid induced-apoptosis of osteoblasts and osteocytes by protecting against endoplasmic reticulum (ER) stress in vitro and in vivo in female mice. Bone 73, 60–68 (2015).
Jilka, R. L. et al. in Apoptosis in Bone Cells in Principles of Bone Biology (eds Bilezikian, J. P. et al.) 237–261 (Academic Press, 2008). This book chapter describes in detail the mechanisms that lead to or prevent apoptosis of osteoblasts, osteocytes and osteoclasts, and the consequences for the skeleton.
Plotkin, L. I., Bivi, N. & Bellido, T. A bisphosphonate that does not affect osteoclasts prevents osteoblast and osteocyte apoptosis and the loss of bone strength induced by glucocorticoids in mice. Bone 49, 122–127 (2011).
Kousteni, S. et al. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298, 843–846 (2002).
Bellido, T. et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J. Biol. Chem. 278, 50259–50272 (2003).
Jilka, R. L. et al. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J. Clin. Invest. 104, 439–446 (1999).
Lin, C. et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β-catenin signaling. J. Bone Miner. Res. 24, 1651–1661 (2009).
Bodine, P. V. Wnt signaling control of bone cell apoptosis. Cell Res. 18, 248–253 (2008).
Glantschnig, H. et al. A rate-limiting role for Dickkopf-1 in bone formation and the remediation of bone loss in mouse and primate models of postmenopausal osteoporosis by an experimental therapeutic antibody. J. Pharmacol. Exp. Ther. 338, 568–578 (2011).
Glantschnig, H. et al. Generation and selection of novel fully human monoclonal antibodies that neutralize Dickkopf-1 (DKK1) inhibitory function in vitro and increase bone mass in vivo. J. Biol. Chem. 285, 40135–40147 (2010).
Bodine, P. V. et al. A small molecule inhibitor of the Wnt antagonist secreted frizzled-related protein-1 stimulates bone formation. Bone 44, 1063–1068 (2009).
Jelinek, T. & Hajek, R. Monoclonal antibodies — a new era in the treatment of multiple myeloma. Blood Rev. 30, 101–110 (2015).
Costa, A. G. & Bilezikian, J. P. Sclerostin: therapeutic horizons based upon its actions. Curr. Osteoporos. Rep. 10, 64–72 (2012).
McClung, M. R. et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 370, 412–420 (2014).
Van Bezooijen, R. L. et al. Sclerostin in mineralized matrices and van Buchem disease. J. Dent. Res. 88, 569–574 (2009).
Van Bezooijen, R. L. et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J. Exp. Med. 199, 805–814 (2004).
Roudier, M. et al. Sclerostin is expressed in articular cartilage but loss or inhibition does not affect cartilage remodeling during aging or following mechanical injury. Arthritis Rheum. 65, 721–731 (2013).
Bouaziz, W. et al. Loss of sclerostin promotes osteoarthritis in mice via β-catenin-dependent and -independent Wnt pathways. Arthritis Res. Ther. 17, 24 (2015).
Drake, M. T. & Farr, J. N. Inhibitors of sclerostin: emerging concepts. Curr. Opin. Rheumatol. 26, 447–452 (2014).
Imel, E. A. et al. Prolonged correction of serum phosphorus in adults with X-linked hypophosphatemia using monthly doses of KRN23. J. Clin. Endocrinol. Metab. 100, 2565–2573 (2015).
Aono, Y. et al. Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J. Bone Miner. Res. 24, 1879–1888 (2009).
Wohrle, S. et al. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J. Bone Miner. Res. 28, 899–911 (2013).
Schwarz, E. M. & Ritchlin, C. T. Clinical development of anti-RANKL therapy. Arthritis Res. Ther. 9, S7 (2007).
Tanaka, S., Nakamura, K., Takahasi, N. & Suda, T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL–RANK signaling system. Immunol. Rev. 208, 30–49 (2005).
Reid, I. R. Denosumab after 8 years. Osteoporos. Int. 26, 2759–2761 (2015).
Sibai, T., Morgan, E. F. & Einhorn, T. A. Anabolic agents and bone quality. Clin. Orthop. Relat. Res. 469, 2215–2224 (2011).
Li, M. et al. Osteopenia and impaired fracture healing in aged EP4 receptor knockout mice. Bone 37, 46–54 (2005).
Ke, H. Z. et al. A nonprostanoid EP4 receptor selective prostaglandin E2 agonist restores bone mass and strength in aged, ovariectomized rats. J. Bone Miner. Res. 21, 565–575 (2006).
Liu, C. C. et al. Novel EP4 receptor agonist-bisphosphonate conjugate drug (C1) promotes bone formation and improves vertebral mechanical properties in the ovariectomized rat model of postmenopausal bone loss. J. Bone Miner. Res. 30, 670–680 (2015).
Li, M., Thompson, D. D. & Paralkar, V. M. Prostaglandin E2 receptors in bone formation. Int. Orthop. 31, 767–772 (2007).
Acknowledgements
L.I.P. acknowledges support from the National Institutes of Health (grants R01-AR053643 and R01-AR067210). T.B. acknowledges support from the National Institutes of Health (grants R01-AR059357, R01-DK076007 and S10-RR023710) and the Veteran's Administration Merit Review 1 I01 (grant BX002104-01).
Author information
Authors and Affiliations
Contributions
L.I.P. and T.B. made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Osteocytes
-
Former osteoblasts embedded in the bone matrix that regulate the formation and function of osteoblasts and osteoclasts.
- Bone
-
Specialized connective tissue composed of a mineralized collagen matrix.
- Osteoblasts
-
Bone forming cells.
- Osteoclasts
-
Bone resorbing cells.
- Bone formation
-
The process by which osteoblasts lay down osteoid and its subsequent mineralization.
- Bone resorption
-
The process by which osteoclasts remove mineralized bone.
- Mechanical stimulation
-
Physical force applied to the skeleton that activates intracellular signalling in osteocytes, which in turn transduce these signals into molecular mediators that control the processes of bone formation and resorption.
- Sclerostin
-
Protein produced by mature osteocytes that inhibits canonical Wnt signalling and decreases bone formation.
- Parathyroid hormone
-
Calcium-regulating hormone that is used as a pharmacotherapy to treat osteoporosis and other bone diseases.
- Apoptosis
-
A process by which cells undergo programmed cell death.
- Osteoid
-
Nonmineralized collagenous bone matrix.
- Bisphosphonates
-
Drugs used to treat conditions with low bone mass and increased fracture risk.
- Canonical Wnt signalling
-
Signalling pathway that results from activation of the LRP5/6 and frizzled co-receptors, leading to accumulation of β-catenin, its nuclear translocation and transcription of specific target genes that affect bone formation and resorption.
- Glucocorticoids
-
Hormones secreted from the adrenal gland and used as immunomodulators that profoundly affect bone, decreasing bone mass and increasing the risk of fractures.
Rights and permissions
About this article
Cite this article
Plotkin, L., Bellido, T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat Rev Endocrinol 12, 593–605 (2016). https://doi.org/10.1038/nrendo.2016.71
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.71
This article is cited by
-
Rescuing SERCA2 pump deficiency improves bone mechano-responsiveness in type 2 diabetes by shaping osteocyte calcium dynamics
Nature Communications (2024)
-
Discovery and optimized extraction of the anti-osteoclastic agent epicatechin-7-O-β-D-apiofuranoside from Ulmus macrocarpa Hance bark
Scientific Reports (2023)
-
Resveratrol protects osteocytes against oxidative stress in ovariectomized rats through AMPK/JNK1-dependent pathway leading to promotion of autophagy and inhibition of apoptosis
Cell Death Discovery (2023)
-
Messages from the Mineral: How Bone Cells Communicate with Other Tissues
Calcified Tissue International (2023)
-
Contributions of Resin Cast Etching to Visualising the Osteocyte Lacuno-Canalicular Network Architecture in Bone Biology and Tissue Engineering
Calcified Tissue International (2023)